Preparation method of nitrobenzoate
A technology of nitrobenzoate and fuming nitric acid, applied in the field of pesticides, can solve the problems of high processing cost, unfriendly environment, large amount of waste acid generated, etc., and achieves good reaction selectivity, easy control of reaction temperature, and high yield. rate increase effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
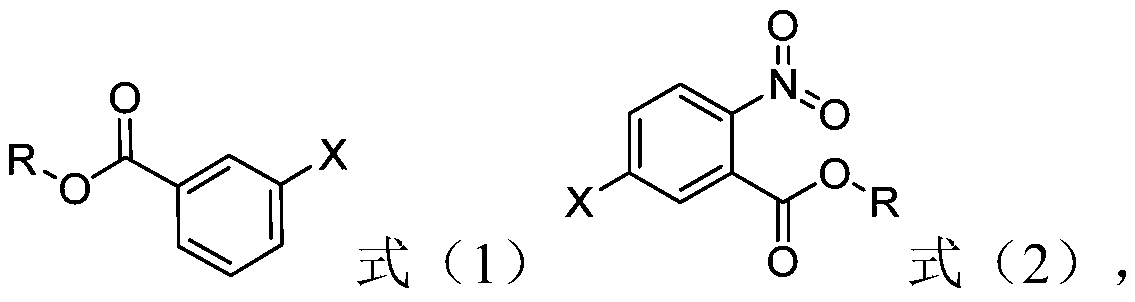
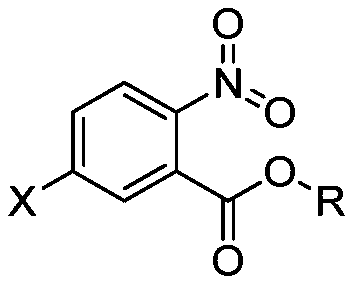
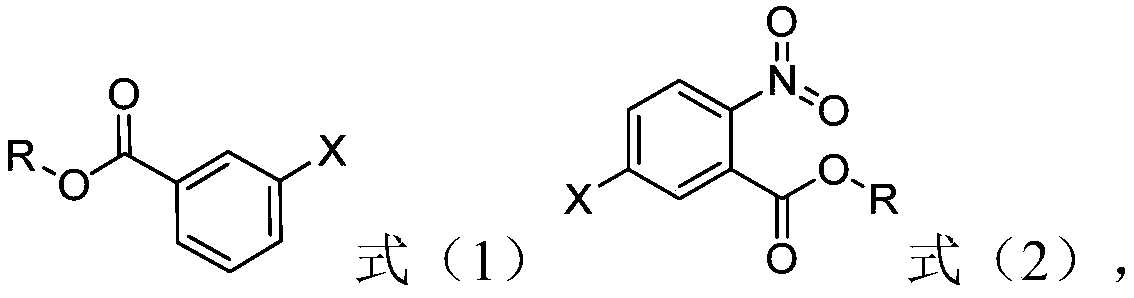
[0023] The preparation method of the nitrobenzoate of structure shown in formula (2) provided by the invention comprises: in the presence of halogenated hydrocarbon organic solvent, the compound of structure shown in formula (1) and fuming nitric acid, fuming The step of contacting sulfuric acid and acetic anhydride,
[0024]
[0025] In formula (1) and formula (2), X represents a halogen, and R represents an alkyl group having 1-8 carbon atoms.
[0026] In the present invention, with the halogenated benzoic acid ester of structure shown in formula (1) as raw material, with halogenated hydrocarbon organic solvent as solvent, with fuming nitric acid as nitrating reagent, fuming sulfuric acid as dehydrating agent, acetic anhydride Nitrification is carried out for reactive dehydration.
[0027] According to the method of the present invention, preferably, X is F, Cl or Br.
[0028] In the present invention, the "alkyl group having 1 to 8 carbon atoms" may be chain, branched ...
Embodiment 1
[0042] Compounding acid: In a 250ml flask, add fuming sulfuric acid (1.8mol, 3 equivalents), stir and cool down to 10°C, add fuming nitric acid (0.7mol, 1.2 equivalents) dropwise, control the temperature below 10°C, and cool after mixing spare.
[0043]Add raw materials methyl m-chlorobenzoate (0.6mol, 1 equivalent), acetic anhydride (0.6mol, 1 equivalent), 240g dichloroethane in the reaction flask, stir and cool down to minus 10°C, add mixed acid dropwise, and the dropping temperature does not exceed -5°C, heat up to 0°C, keep at 0°C for 2h. Separate the layers, separate the organic phase (upper layer), drop the acid layer into 100g of ice water to quench, add 240g of dichloroethane for extraction, separate the waste acid phase and combine the organic phase, add 100g of water to wash, separate the water phase; add the organic phase to Wash in the alkaline washing solution made of 10g sodium bicarbonate, 10g sodium carbonate and 180g water, separate the alkaline aqueous phase...
Embodiment 2
[0047] Compounding acid: In a 250ml flask, add fuming sulfuric acid (3mol, 5 equivalents), stir and cool down to 10°C, add fuming nitric acid (0.6mol, 1.2 equivalents) dropwise, control the temperature below 10°C, prepare and cool for later use .
[0048] Add raw materials isopropyl m-chlorobenzoate (0.6mol, 1 equivalent), acetic anhydride (0.72mol, 1.2 equivalents), 240g dichloroethane in the reaction flask, stir and cool down to minus 10°C, add mixed acid dropwise, the dropping temperature is constant When the temperature exceeds minus 5°C, the temperature is raised to 0°C, and the temperature is kept at 0°C for 2 hours. Separate the layers, separate the organic phase (upper layer), drop the acid layer into 100g of ice water to quench, add 240g of dichloroethane for extraction, separate the waste acid phase and combine the organic phase, add 100g of water to wash, separate the water phase; add the organic phase to Wash in the alkaline washing solution made of 10g sodium bic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com