Method for analyzing Roxadustat related substances
A technology for roxadustat and related substances, which is applied in the field of analysis of roxadustat related substances, and can solve problems such as methods for determining related substances of roxadustat that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Choice of diluent.
[0042] Purpose of the test: Due to the difference in polarity of different impurities, different polar solvents can dissolve impurities differently. Therefore, in order to ensure that the impurities in the sample are completely dissolved, the dilution solution is studied.
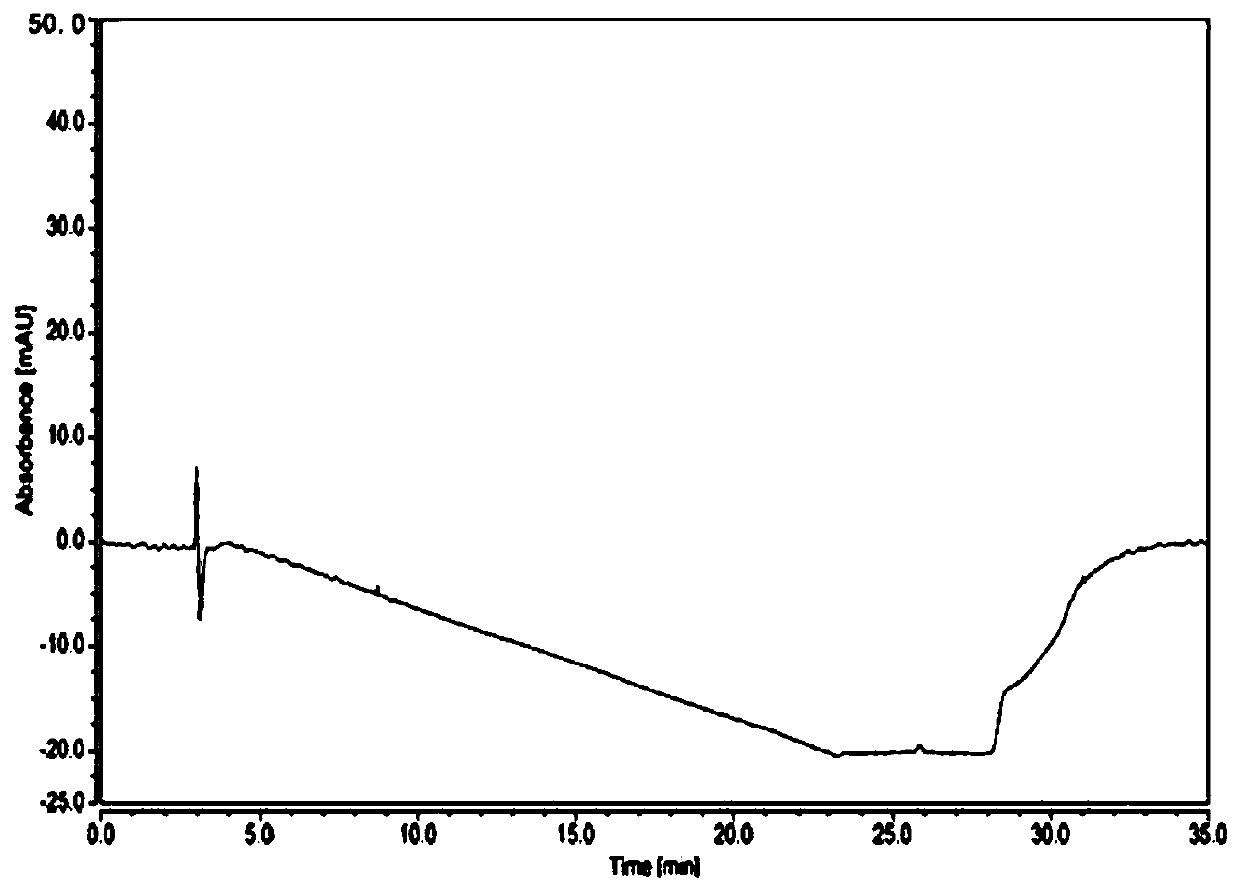
[0043] Test process: Take roxadustat, impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity H, and impurity I, and dilute them with acetonitrile respectively to prepare a solution with a concentration of 0.2 mg / ml, wherein Impurity D, impurity H, and impurity I cannot be completely dissolved. Use a mixture of acetonitrile and water with a volume ratio of 4:1 as the diluent, then roxadustat and its related substances can be completely dissolved, see figure 1 .
[0044] Impurity A is 4-hydroxyl-N, N-dimethyl-7-phenoxyisoquinoline-3-carboxamide; impurity B is [4-hydroxyl-1-(hydroxymethyl)-7-phenoxy Isoquinolin-3-yl] (morpholinyl) ketone; Impurity C is [...
Embodiment 2
[0047] Column screening.
[0048] Purpose of the test: to select a suitable chromatographic column to effectively separate roxadustat and related substances.
[0049] Instrument equipment and chromatographic conditions: liquid chromatograph: Thermo UltiMate 3000; ultraviolet variable wavelength detector; gradient elution with 0.1% trifluoroacetic acid aqueous solution-acetonitrile as mobile phase, see Table 1; column temperature 30°C; detection wavelength 230nm; flow rate 1ml per minute; injection volume 10μl;
[0050] Table 1 Gradient elution program
[0051]
[0052] Sample preparation: take roxadustat, impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity H, and impurity I, and use acetonitrile and water with a volume ratio of 4:1 as the diluent Diluted to prepare a solution containing 0.2 mg of roxadustat per 1 ml and 0.2 μg of each impurity. See Table 2 for column selection.
[0053] Table 2 Screened column list
[0054] serial ...
Embodiment 3
[0057] Impurity localization study
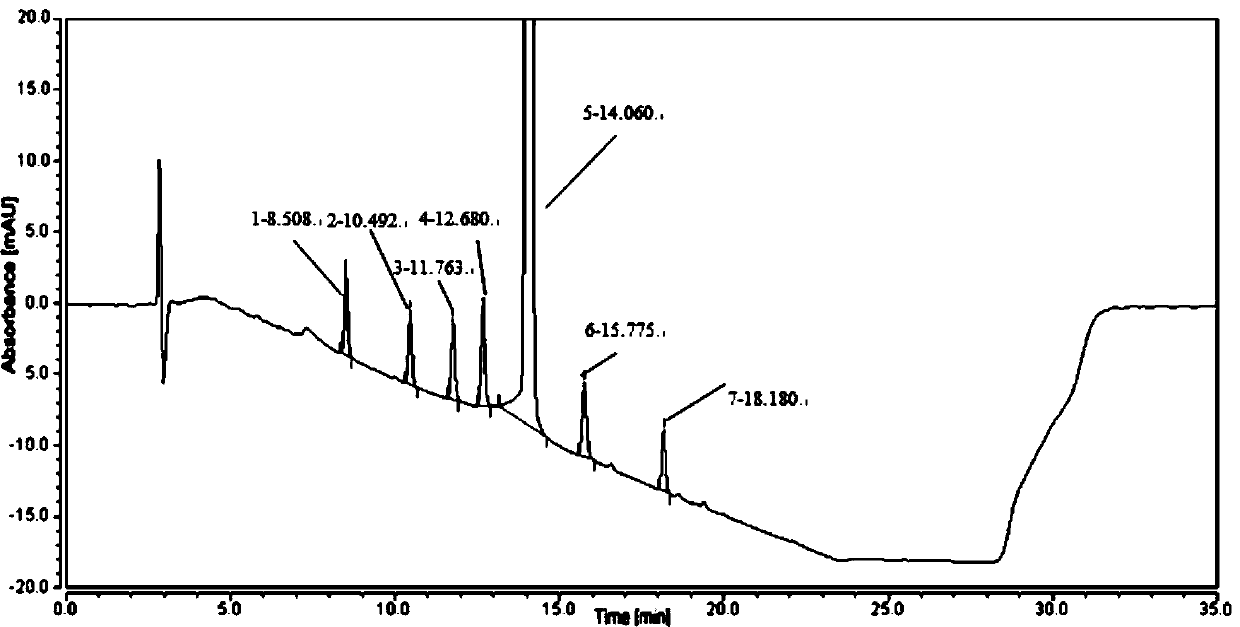
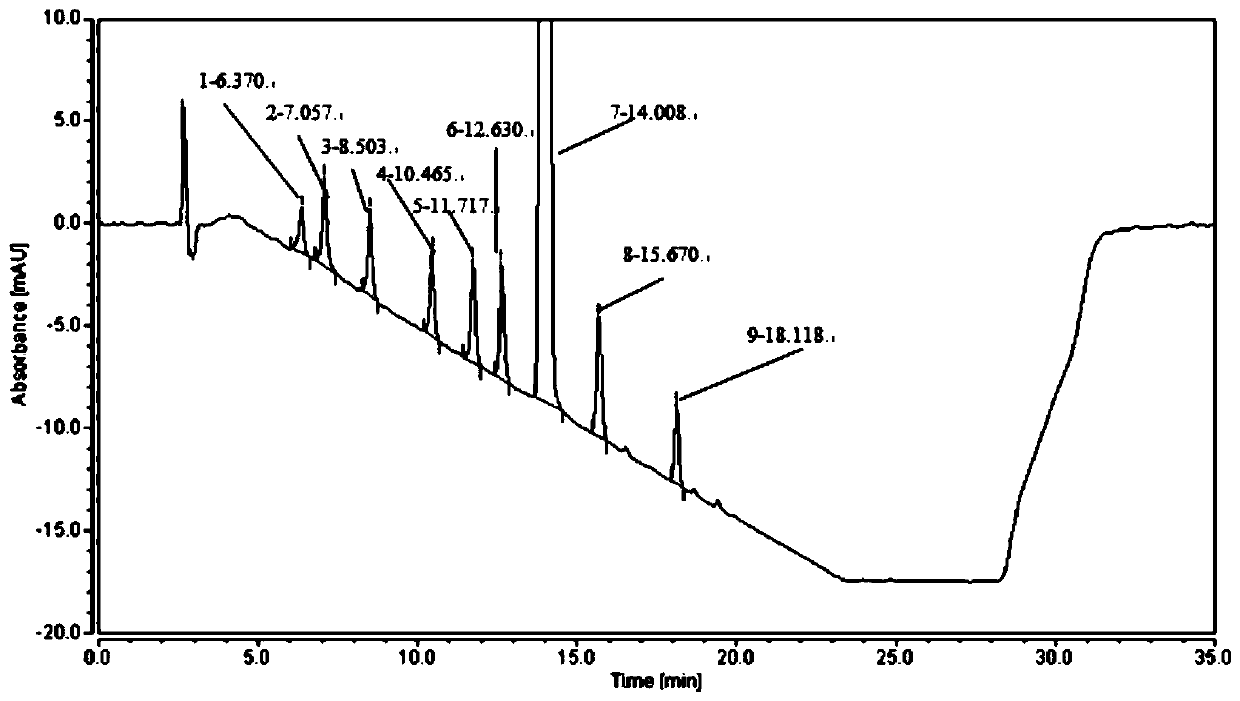
[0058] Purpose of the test: to determine the retention time of roxadustat and each known impurity.
[0059]Instrument equipment and chromatographic conditions: liquid chromatograph: Thermo UltiMate 3000; UV variable wavelength detector; chromatographic column: Ultimate XB-C8, 250mm*4.6mm, 3μm; use 0.1% trifluoroacetic acid aqueous solution-acetonitrile as mobile phase Gradient elution, see Table 3; column temperature 30°C; detection wavelength 230nm; flow rate 1ml per minute; injection volume 10μl;
[0060] Table 3 Gradient elution program
[0061]
[0062] Sample preparation: Take roxadustat, impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity H, and impurity I, and dilute them with acetonitrile and water at a volume ratio of 4:1. The solution was diluted, and prepared into solutions containing 0.2 mg of roxadustat and each related substance per 1 ml, respectively, as each positioning solution. Take roxad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com