Preparation method and electrocatalytic application of perovskite electrode material
An electrode material, perovskite technology, applied in the direction of electrodes, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of low optical purity of products, few active sites, small specific surface area of flat electrode, etc., to achieve The effect of low cost, simple preparation method and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] a. Preparation of precursor
[0031] Weigh 1.0825 g (0.0025 mol) La(NO 3 ) 3 ·6H 2 O and 0.4496 g (0.0025 mol) Fe(NO 3 ) 3 9H2 O was dissolved in 50 mL of distilled water to form a transparent aqueous solution. After stirring for 10 min, 1.2608 g (0.006 mol) of citric acid monohydrate was added. After stirring for 30 min, the solvent was evaporated in a water bath at 85 °C to obtain a wet gel Dry at 180 °C for 12 h to obtain a fluffy powder as a precursor.
[0032] b. Preparation of perovskite electrode materials
[0033] Grind the precursor prepared above and put it into a crucible, cover it and place it in a muffle furnace, and bake it at 500°C for 5 hours to obtain the product LaFeO 3 Perovskite electrode materials (labeled as LaFeO 3 500).
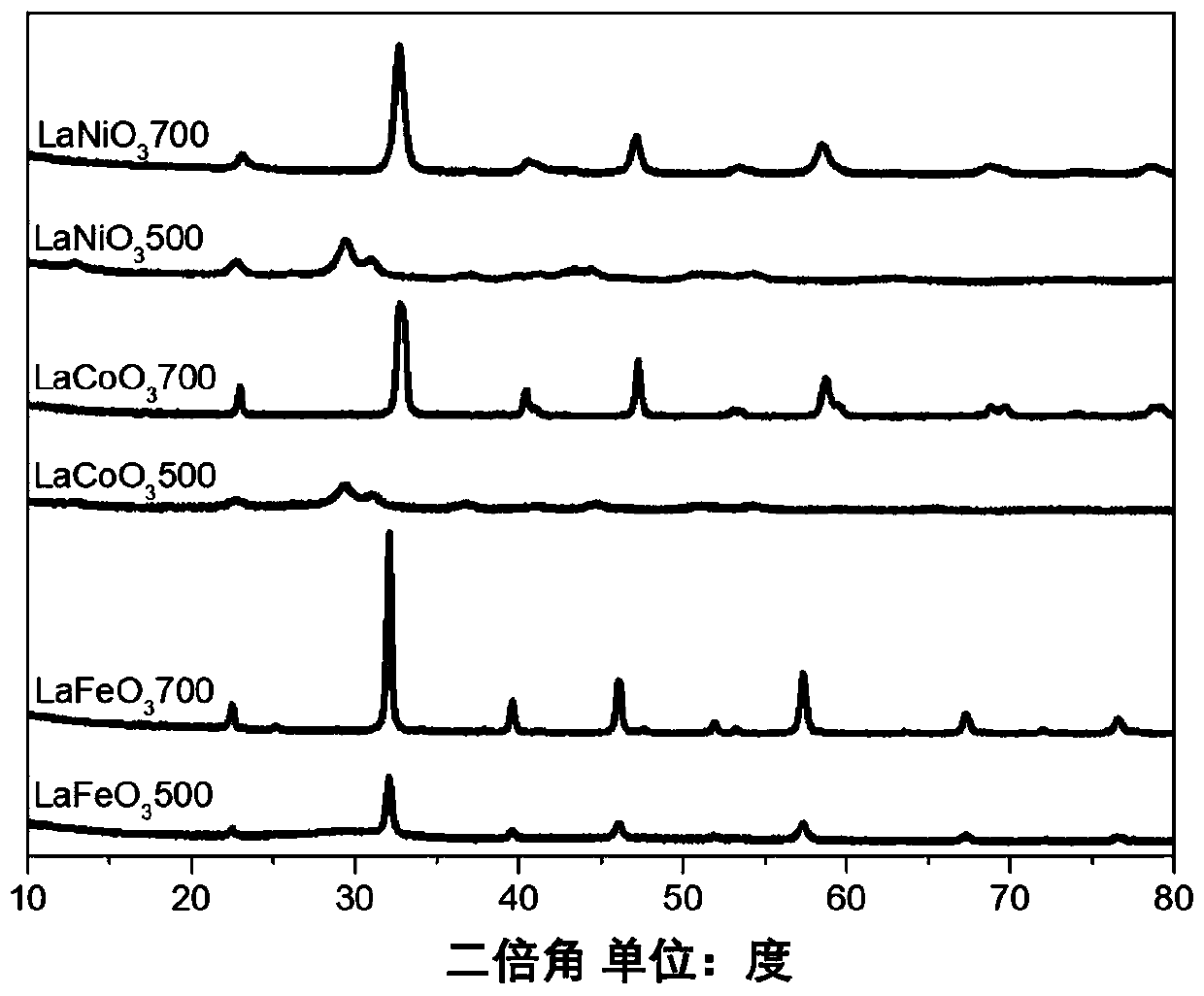
[0034] See attached figure 1 , the above product was characterized by XRD (LaFeO 3 500 curve), the electrode material conforms to the characteristic diffraction peak of JCPDs card no.75-0541 perovskite, indicating that...
Embodiment 2
[0037] a. Preparation of precursor
[0038] With the a step of embodiment 1.
[0039] b. Preparation of perovskite electrode materials
[0040] Grind the precursor prepared above and put it into a crucible, cover it and place it in a muffle furnace, and bake it at 700 °C for 5 hours to obtain the product LaFeO 3 Perovskite electrode materials (labeled as LaFeO 3 700).
[0041] See attached figure 1 , the above product was characterized by XRD (LaFeO 3 700 curve), the electrode material conforms to the characteristic diffraction peak of JCPDs card no.75-0541 perovskite, indicating that a perovskite material with a better crystal form is formed.
[0042] See attached image 3 , the above product was characterized by scanning electron microscope, perovskite LaFeO 3 700 particles dispersed evenly.
Embodiment 3
[0044] a. Preparation of precursor
[0045] Weigh 1.0825 g (0.0025 mol) La(NO 3 ) 3 ·6H 2 O and 1.0000 g (0.0025 mol) Co(NO 3 ) 2 ·6H 2 O was dissolved in 50 mL of distilled water to form a transparent aqueous solution. After stirring for 10 min, 1.2608 g (0.006 mol) of citric acid monohydrate was added. After stirring for 30 min, the solvent was evaporated in a water bath at 85 °C. The obtained wet gel was Dry at 180°C for 12 h to obtain a fluffy powder as a precursor.
[0046] b. Preparation of perovskite electrode materials
[0047] Grind the precursor prepared above and put it into a crucible, cover it and place it in a muffle furnace, and bake it at 500 °C for 5 hours to obtain the product LaCoO 3 to the perovskite electrode material (labeled as LaCoO 3 500).
[0048] See attached figure 1 , the above product was characterized by XRD (LaCoO 3 500 curve), the electrode material does not have the characteristic diffraction peaks of perovskite, indicating that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com