Preparation method of 2-alkyl-2-aminopropionate hydrochloride
An aminopropionate, hydrochloride technology, applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation and other directions, can solve the problems of high cost, incomplete reaction of raw materials, high price of BOC acid anhydride, etc. The effect of high product yield and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
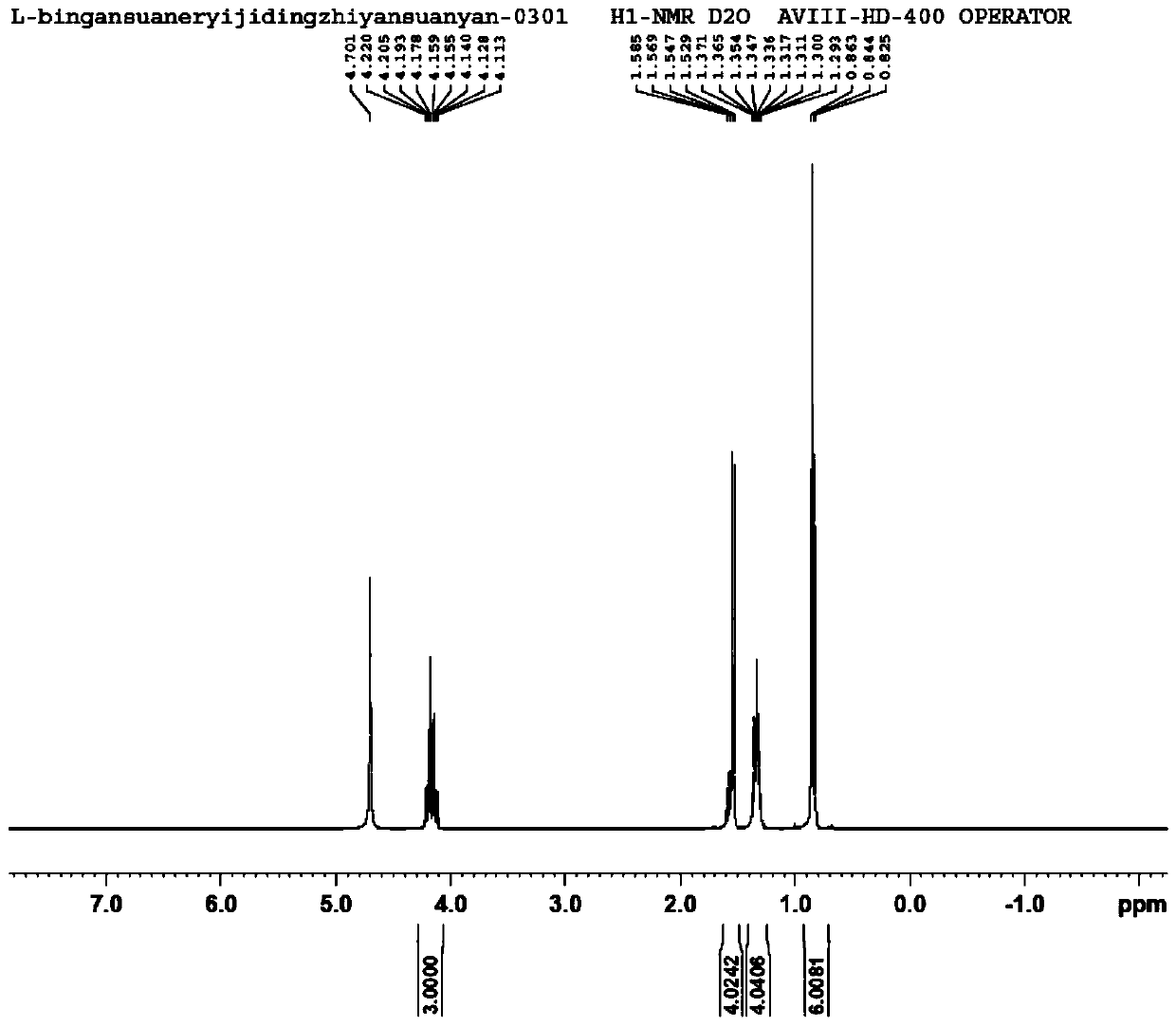
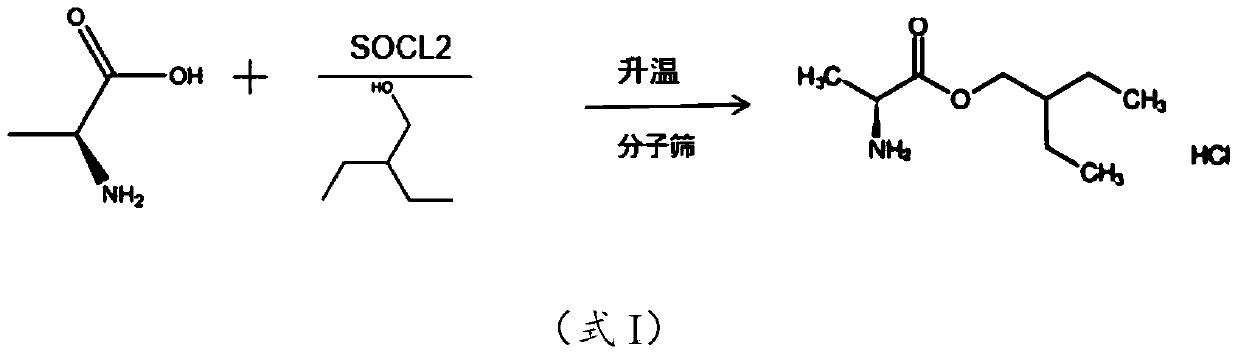
[0014] The embodiment of the present application provides a preparation method of 2-alkyl-2-aminopropionate hydrochloride, which includes: mixing L-alanine and alkyl alcohol at a temperature of -10 to 5°C Add thionyl chloride dropwise into the solution, heat preservation treatment to hydrochloride the amino group of L-alanine to obtain a reaction stock solution. The reaction stock solution is heated to 75-85°C in the presence of molecular sieves to carry out esterification reaction to obtain a reaction solution containing 2-alkyl-2-aminopropionate hydrochloride.
[0015] In the examples of this application, the amino group of L-alanine was hydrochloridized first by using thionyl chloride, and the esterification reaction during hydrochlorication was avoided under the temperature condition of -10~5°C, effectively avoiding the occurrence of L-alanine The amino group of the amino acid undergoes an amidation reaction in the esterification reaction, and there is no need to use BOC a...
Embodiment 1
[0036] A preparation method of (S)-2-ethylbutyl-2-aminopropionate hydrochloride, comprising:
[0037] S1. Add 100g of L-alanine to 450mL of 2-ethyl-1-butanol, lower the temperature of the system to below 0°C, and drop 160g of thionyl chloride into the mixture. During the dropwise addition, the temperature of the system was controlled below 5°C, and after the dropwise addition was completed, the temperature was kept below 5°C for 30 minutes to obtain the reaction stock solution.
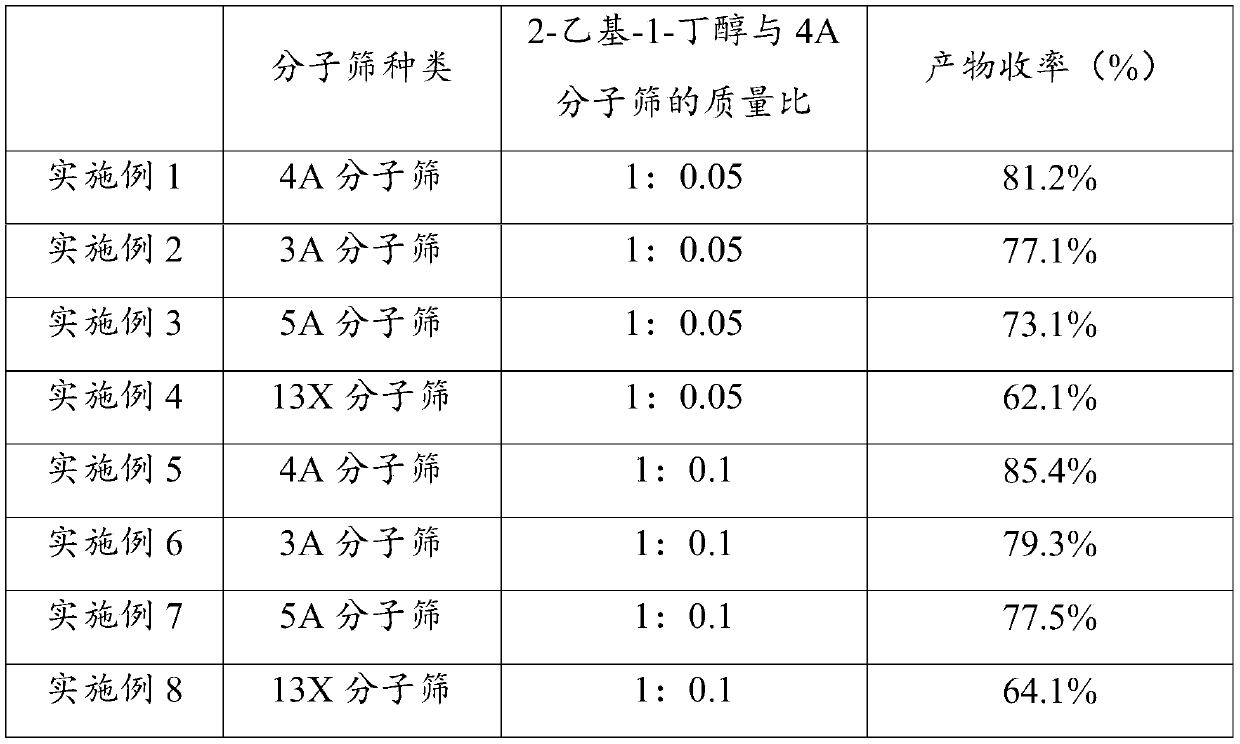
[0038] S2. Add 4A molecular sieve to the reaction stock solution, the mass ratio of 2-ethyl-1-butanol to 4A molecular sieve is 1:0.05; then heat the reaction stock solution to 80°C for 10h to obtain (S)-2-Ethyl The reaction solution of butyl-2-aminopropionate hydrochloride.
[0039] S3. Filter the reaction solution to remove the molecular sieve, then concentrate the reaction solution to a slurry, add 100mL petroleum ether to crystallize, filter and dry the crystal, (S)-2-ethylbutyl-2-aminopropionate ...
Embodiment 2
[0041] A preparation method of (S)-2-ethylbutyl-2-aminopropionate hydrochloride, comprising:
[0042] S1. Add 100g of L-alanine to 450mL of 2-ethyl-1-butanol, lower the temperature of the system to below 0°C, and drop 160g of thionyl chloride into the mixture. During the dropwise addition, the temperature of the system was controlled below 5°C, and after the dropwise addition was completed, the temperature was kept below 5°C for 30 minutes to obtain the reaction stock solution.
[0043] S2. Add 3A molecular sieve to the reaction stock solution, the mass ratio of 2-ethyl-1-butanol to 3A molecular sieve is 1:0.05; then heat the reaction stock solution to 80°C for 10h to obtain (S)-2-ethyl The reaction solution of butyl-2-aminopropionate hydrochloride.
[0044] S3. Filter the reaction solution to remove the molecular sieve, then concentrate the reaction solution to a slurry, add 100mL petroleum ether to crystallize, filter and dry the crystal to obtain (S)-2-ethylbutyl-2-aminopr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com