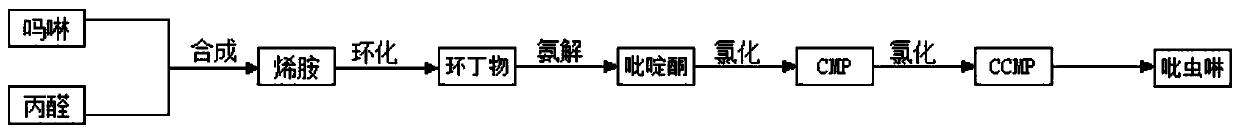
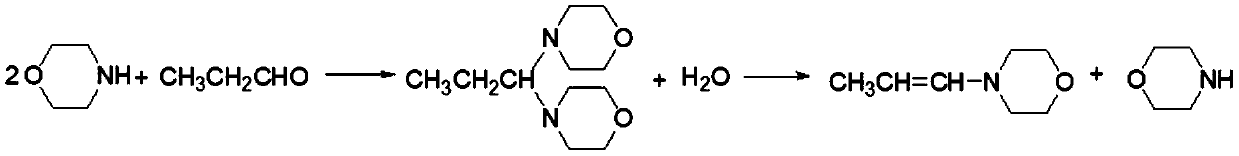
Method for synthesizing imidacloprid intermediate morpholinyl propylene by micro-channel reactor
A micro-channel reactor, morpholine propylene technology, applied in the direction of organic chemistry, can solve the problems of low production cost, poor heat transfer effect, high energy consumption, etc., to eliminate back mixing, improve reaction selectivity, and accurate temperature control. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Pump morpholine and n-propionaldehyde into the microchannel reactor according to the molar ratio of 10.0:1, and react at a reaction temperature of 20°C and a reaction pressure of 0.2MPa. By adjusting the flow rate of the feed pump, the residence time of the reaction material is controlled to 200s. The flow rate of the mixed solution is 0.12ml / s. The product was incubated at 20°C for 1.5hr, and after the incubation was completed, it was dehydrated under negative pressure at a vacuum degree of 3kPa, and the final liquid temperature was 80°C. The dehydrated product was subjected to negative pressure rectification at a vacuum degree of 2.5kPa, and the liquid temperature at the end point was 130°C to obtain the enamine product for the next reaction. The finished product of enamine was analyzed, and the enamine yield was 89.3% based on n-propionaldehyde.
Embodiment 2
[0029] Pump morpholine and n-propionaldehyde into the microchannel reactor at a molar ratio of 4.0:1, and react at a reaction temperature of 40°C and a reaction pressure of 2.0 MPa. The residence time of the reaction materials is controlled to be 10s by adjusting the flow rate of the feed pump. The flow rate of the mixed solution is 2.4ml / s. The product was incubated at 40°C for 3 hours, and after the incubation was completed, it was dehydrated under negative pressure at a vacuum degree of 0.1kPa, and the liquid temperature at the end point was 40°C. After further dehydration, the product was subjected to negative pressure rectification under a vacuum degree of 2.1kPa, and the liquid temperature at the end point was 120°C to obtain the finished enamine for the next reaction. The finished product of enamine was analyzed, and the enamine yield was 97.6% based on n-propionaldehyde.
Embodiment 3
[0031] Pump morpholine and n-propionaldehyde into the microchannel reactor according to the molar ratio of 2.4:1, and react at a reaction temperature of 50°C and a reaction pressure of 0.8MPa. By adjusting the flow rate of the feed pump, the residence time of the reaction materials is controlled to be 15s. The flow rate of the mixed solution is 1.5ml / s. The product was incubated at 50°C for 2 hours, and after the incubation was completed, it was dehydrated under negative pressure at a vacuum degree of 0.3kPa, and the final liquid temperature was 45°C. The dehydrated product was subjected to negative pressure rectification under a vacuum degree of 1.3kPa, and the liquid temperature at the end point was 105°C to obtain the finished enamine for the next reaction. The enamine finished product was analyzed, and the enamine yield was 99.3% based on n-propionaldehyde.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com