Estrogen receptor targeting radioactive tracer, and preparation method and application thereof
A radiotracer and estrogen receptor technology, which is applied in the field of medical imaging and achieves the effects of low non-specific background, excellent in vivo biological properties, and a simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
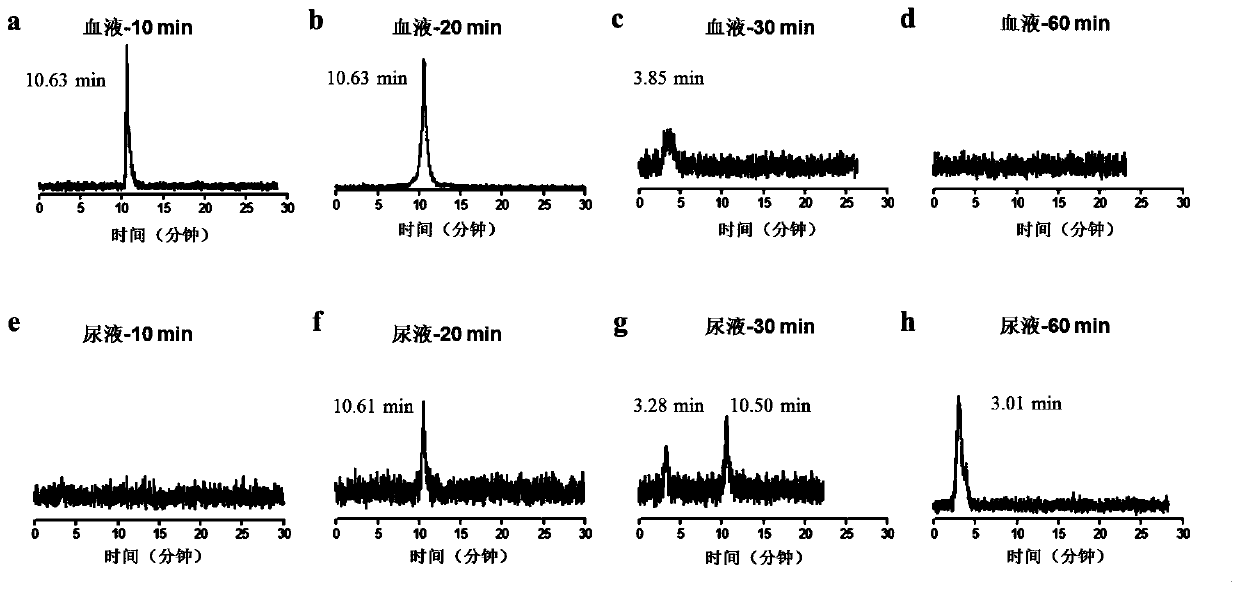
Image
Examples
Embodiment 1
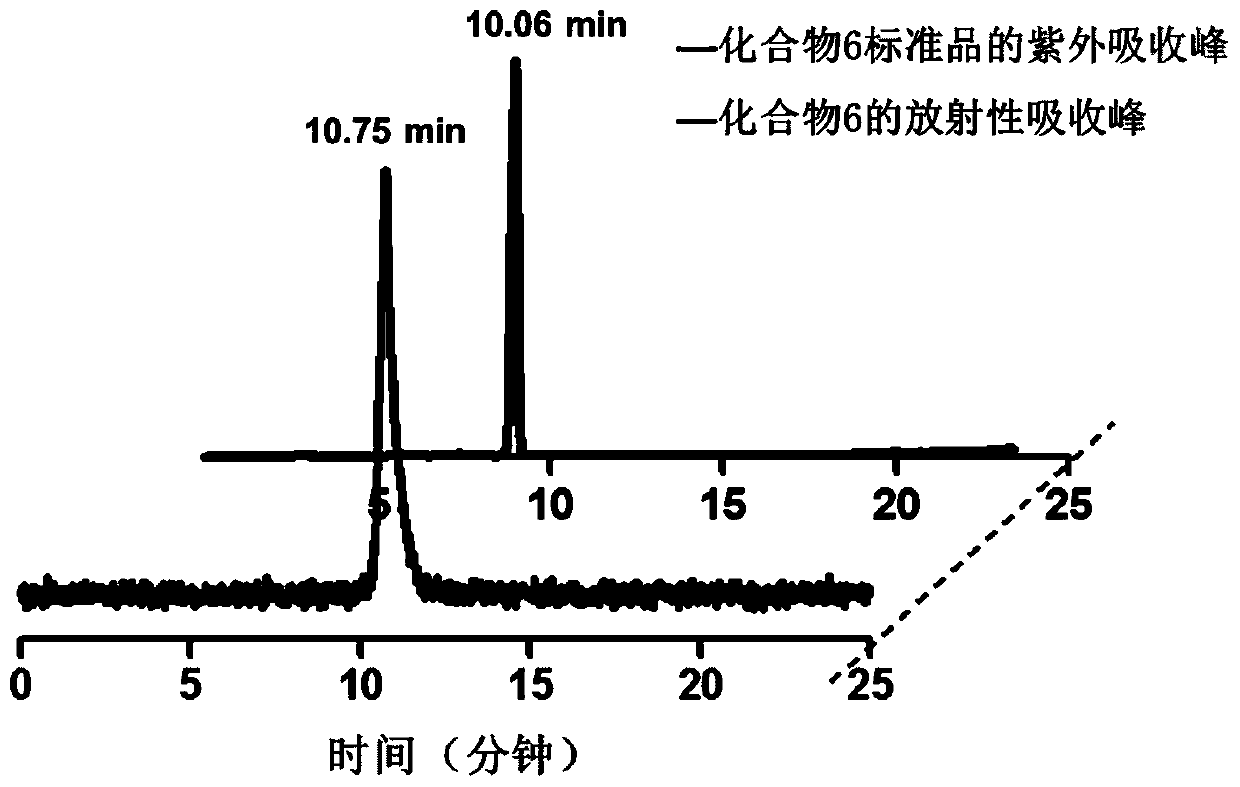
[0042] A preparation method of an estrogen receptor targeting radiotracer (compound 6) of the present embodiment, comprising the following steps:
[0043] 1) Synthesis of compound 2:
[0044] First place the reaction device in an ice-water bath, then dissolve 1.5g of compound 1 and 1.7mL of triethylamine in 10mL of dichloromethane, and finally add 2.29g of p-toluenesulfonyl chloride dissolved in 20mL of dichloromethane, first 0 °C for 1 hour, and then at room temperature for 5 hours. After the reaction, the product was washed with water and the reaction solution was extracted three times with dichloromethane, the organic phase was collected and the solvent was removed by rotary evaporation. The crude product was purified through a silica gel column, and the mobile phase ratio was petroleum ether: ethyl acetate = 2:1. Compound 2 was obtained as pure white crystals.
[0045] 2) Synthesis of compound 3:
[0046] At room temperature, add 0.65g of sodium azide into 10mL of N,N-...
Embodiment 2
[0076] A preparation method of an estrogen receptor targeting radiotracer (compound 8) of the present embodiment, comprising the following steps:
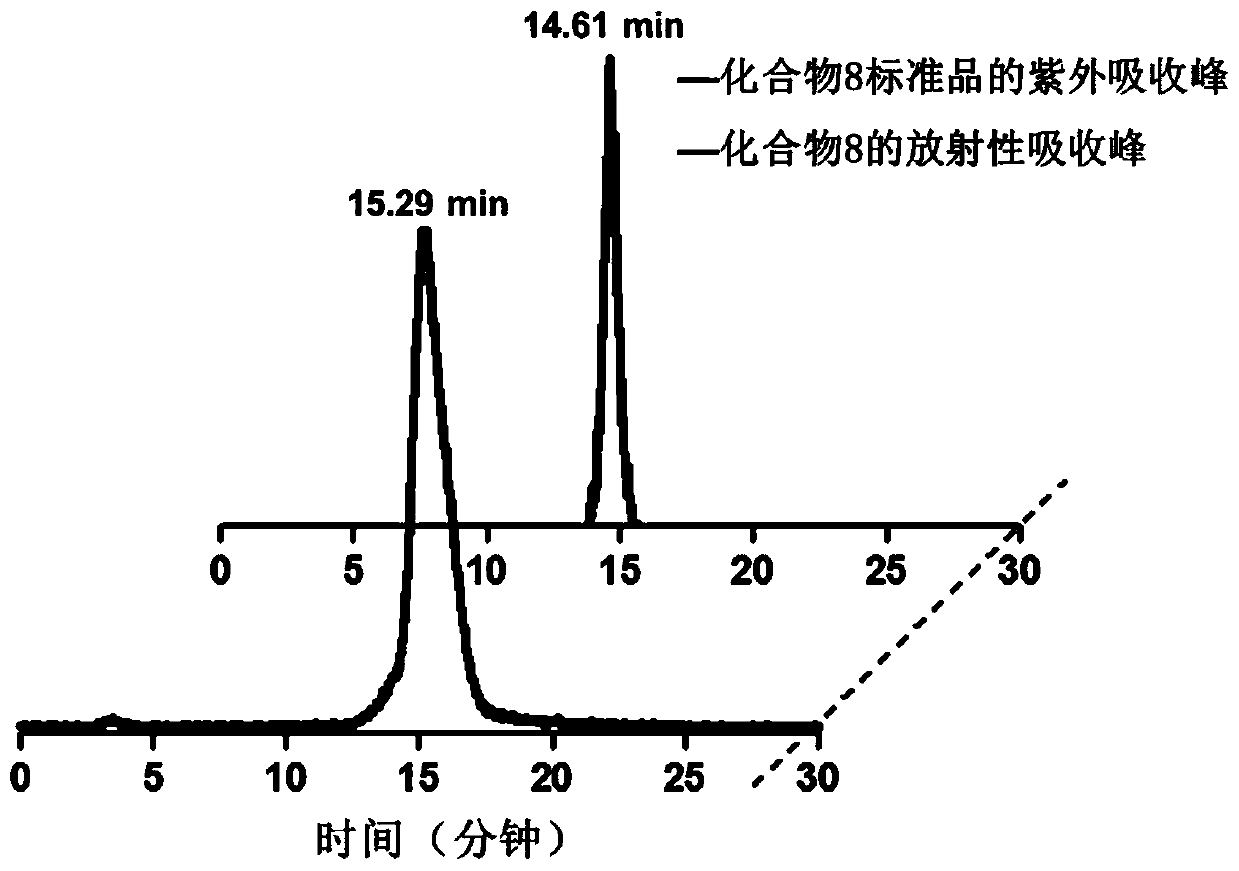
[0077] Add 1mg of compound 7 into a 5mL microwave reaction tube, then add 0.1mL of acetonitrile as a solvent, and finally add 0.1mL of fluorine-18 aqueous solution, and react with microwave at 180°C for 10 minutes. After the reaction was completed, HPLC purification was performed. HPLC conditions are: mobile phase A is water, phase B is acetonitrile: 0 to 10 minutes: 35% B, 10 to 20 minutes: 60% B, 20 to 30 minutes: 35% B; the flow rate is 1 mL / min. The HPLC analysis chart of purified compound 8 and its non-radioactive standard reference compound is as follows: figure 2 As shown, the peak times of the two are similar, which proves the correctness of compound 8 labeling.
[0078] Compound 8 in this example is labeled with the standard reference compound of Compound 6 as a precursor, and the labeling method is simple and easy. Fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com