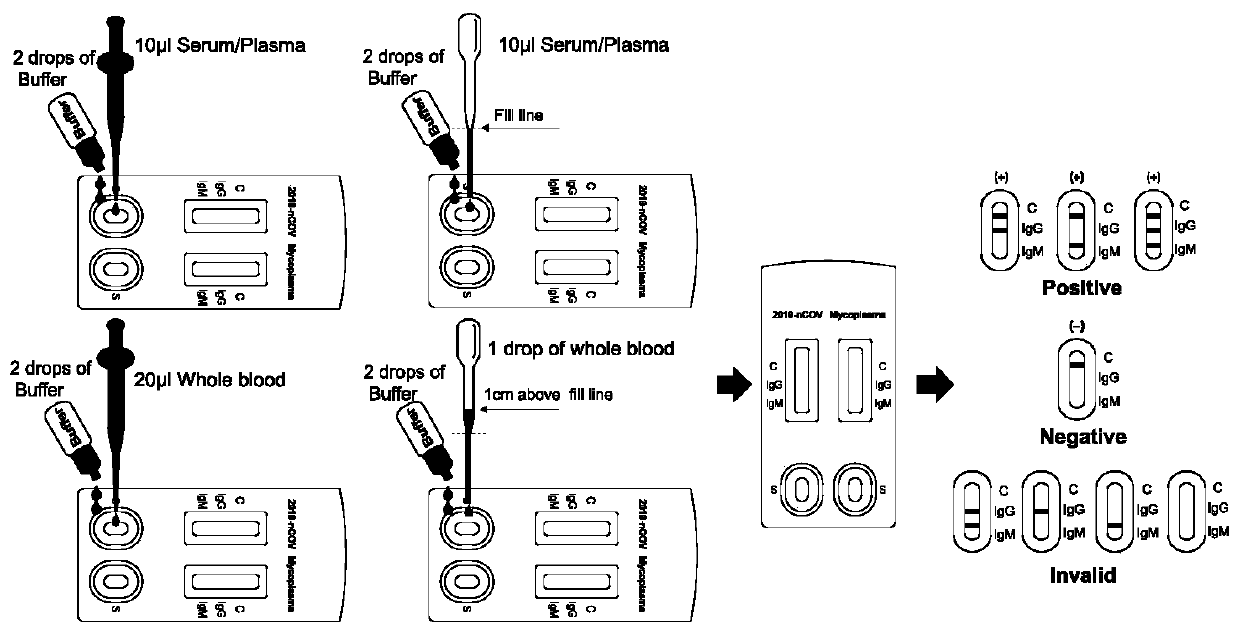
Colloidal gold immunochromatography test paper for combined diagnosis of COVID-19 and mycoplasma pneumoniae and preparation method thereof
A COVID-19, immunochromatographic detection technology, applied in the field of immunodiagnosis, can solve the problem of mycoplasma pneumonia chest X-ray examination is not characteristic, unable to make a diagnosis and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
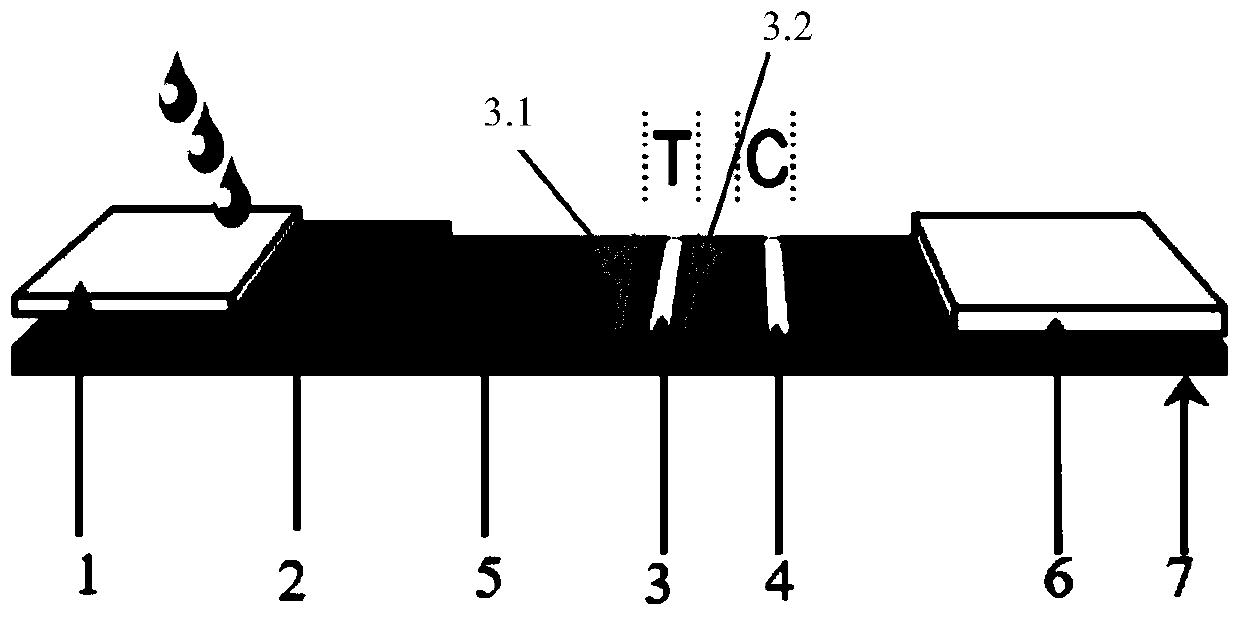
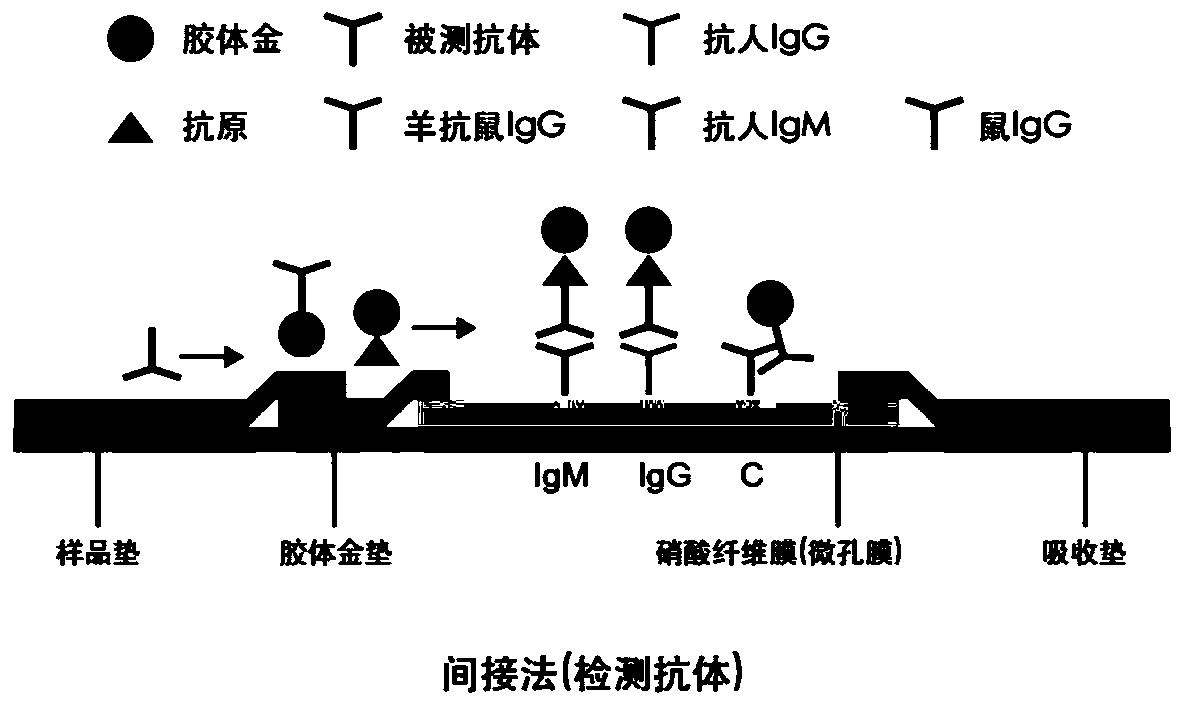
[0056] The mouse anti-human IgM antibody concentration of the detection line IgM coating is 0.1mg / ml, and the mouse anti-human IgG antibody concentration of the detection line IgG coating is 0.1mg / ml; The quality control line C4 coating The goat anti-mouse IgG antibody concentration was 0.5mg / ml. The colloidal gold conjugate treatment pad 2 is coated with a new coronavirus N protein recombinant antigen-colloidal gold conjugate, and the diameter of the new coronavirus N protein recombinant antigen-colloidal gold conjugate is 40nm-100nm, and the new coronavirus recombinant antigen The concentration is 1 mg / ml.
[0057] The preparation method of the colloidal gold immunochromatography detection test paper of described joint diagnosis COVID-19 and Mycoplasma pneumoniae, described A detection test paper specifically comprises the following steps:
[0058] A) Preparation of test line T and quality control line C: Dilute the mouse anti-human IgM, mouse anti-human IgG and goat anti-m...
Embodiment 2
[0068] The difference from Example 1 is that the concentration of the mouse anti-human IgM antibody coated by the detection line IgM is 0.4 mg / ml, and the concentration of the mouse anti-human IgG antibody coated by the detection line IgG is 0.3 mg / ml; Quality control line C 4 coated goat anti-mouse IgG antibody concentration is 0.8mg / ml. The colloidal gold conjugate treatment pad 2 is coated with a new coronavirus N protein recombinant antigen-colloidal gold conjugate, and the diameter of the new coronavirus N protein recombinant antigen-colloidal gold conjugate is 40nm-100nm, and the new coronavirus recombinant antigen The concentration is 3 mg / ml.
[0069] The preparation method of the colloidal gold immunochromatography detection test paper of described joint diagnosis COVID-19 and Mycoplasma pneumoniae, described A detection test paper specifically comprises the following steps:
[0070] A) Preparation of test line T and quality control line C: Dilute mouse anti-human Ig...
Embodiment 3
[0080] The difference with Example 1 is that the concentration of the mouse anti-human IgM antibody coated by the detection line IgM is 1.0 mg / ml, and the concentration of the mouse anti-human IgG antibody coated by the detection line IgG is 1.0 mg / ml; The concentration of goat anti-mouse IgG antibody coated with quality control line C4 is 1.2mg / ml. The colloidal gold conjugate treatment pad 2 is coated with a new coronavirus N protein recombinant antigen-colloidal gold conjugate, and the diameter of the new coronavirus N protein recombinant antigen-colloidal gold conjugate is 40nm-100nm, and the new coronavirus recombinant antigen The concentration is 5mg / ml.
[0081] The preparation method of the colloidal gold immunochromatography detection test paper of described joint diagnosis COVID-19 and Mycoplasma pneumoniae, described A detection test paper specifically comprises the following steps:
[0082] A) Preparation of test line T and quality control line C: Dilute the mouse...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com