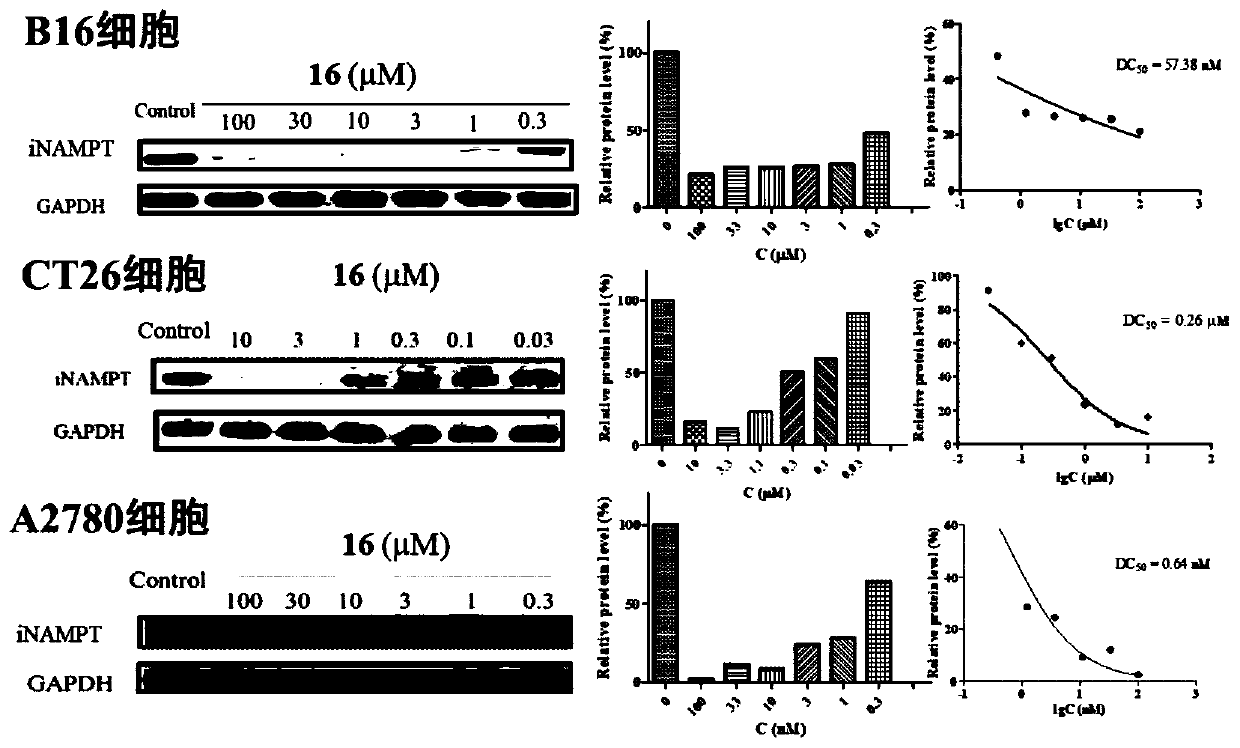
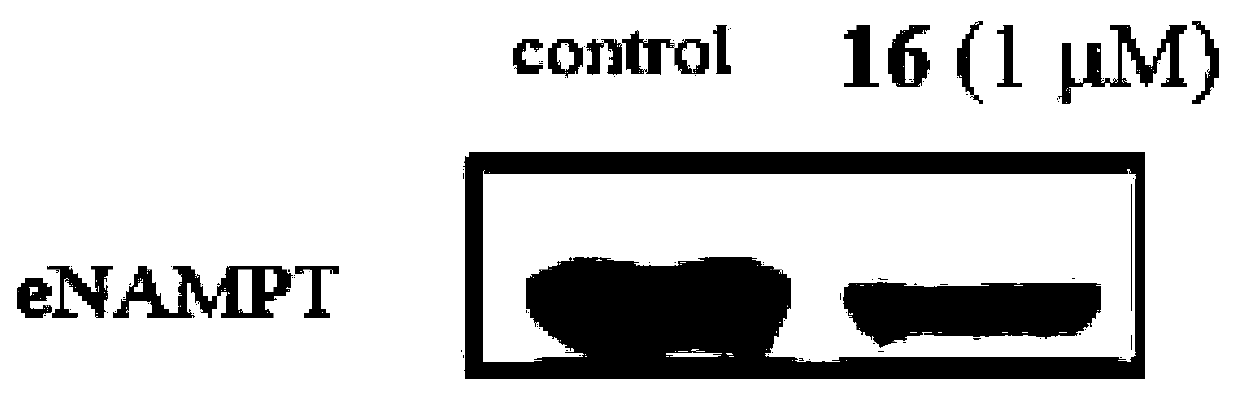
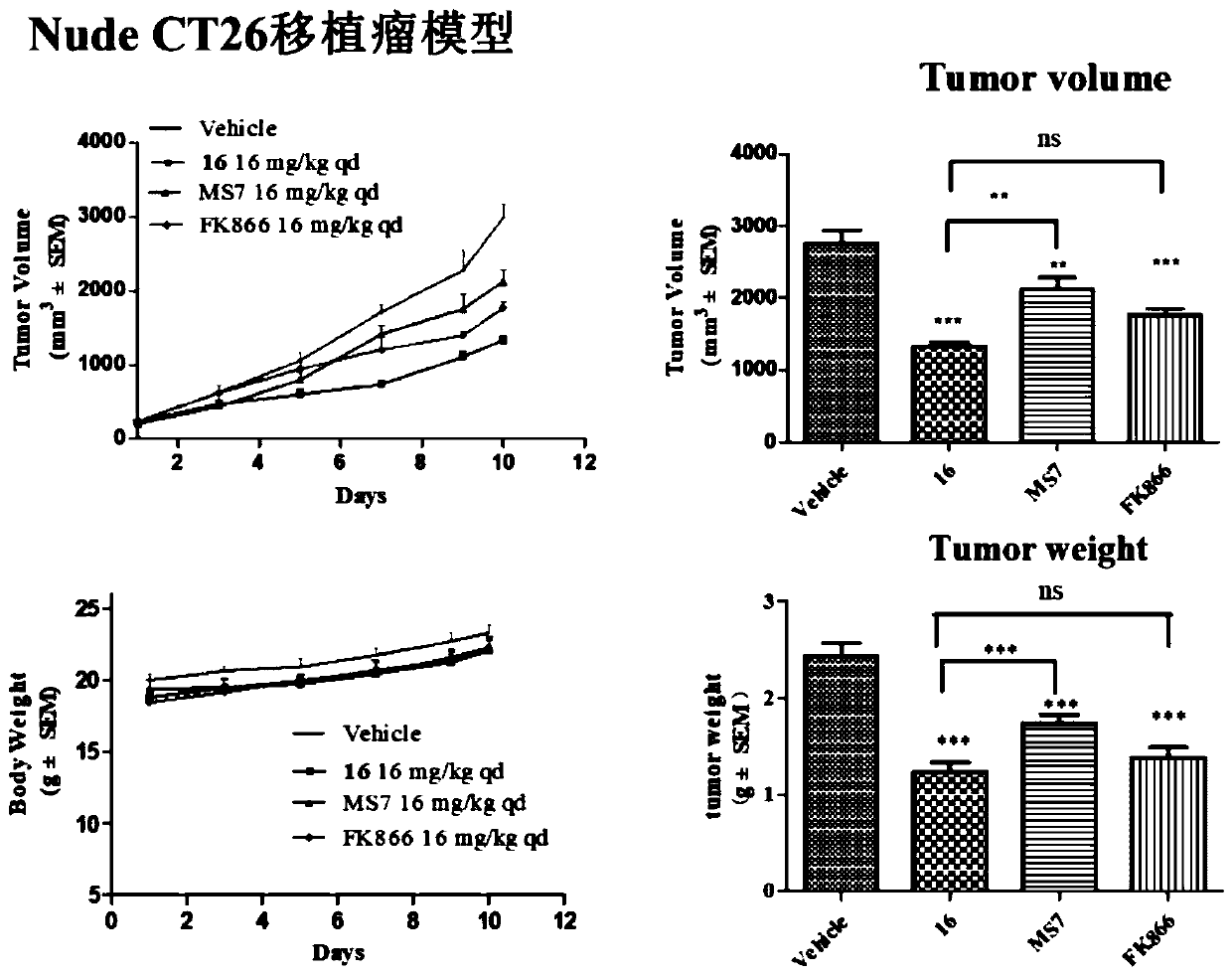
NAMPT protein degradation targeted chimera as well as preparation method and application thereof
A technology of protein degradation and chimera, applied in the field of medicine, can solve problems that have not been reported before
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1 Synthesis of Compound 1
[0132] The preparation method is as follows:
[0133] Step a. Synthesis of tert-butyl 4-((4-nitrophenyl)sulfonyl)piperazine-1-carboxylate:
[0134] Dissolve p-nitrobenzenesulfonyl chloride (2.21g, 10.0mmol) and 1-Boc-piperazine (2.79g, 15.0mmol) in CH 2 Cl 2 (10mL), then add TEA (2mL) dropwise to the solution, stir at room temperature for 1.5h, the reaction solution and 50mL of 2N hydrochloric acid solution are fully mixed, separated, collect the organic phase, evaporate the solvent to obtain a white solid 3.51g, the yield is 95 %. 1 H NMR(600MHz, DMSO-d 6 )δ: 7.78 (d, J = 20.78Hz, 1H), 7.67 (d, J = 20.78Hz, 1H), 3.40-3.37 (m, 4H), 2.89-2.85 (m, 4H), 1.34 (s, 9H) ).
[0135] Step b. Synthesis of tert-butyl 4-((4-aminophenyl)sulfonyl)piperazine-1-carboxylate:
[0136] The compound tert-butyl 4-((4-nitrophenyl)sulfonyl)piperazine-1-carboxylate (3.51g, 9.46mmol) was dissolved in dry CH 2 Cl 2 (30 mL), stirred overnight at room temperature under ...
Embodiment 2~17
[0147] Example 2-17 Synthesis of Compound 2-17
[0148] The operation and feeding are the same as in Example 1.
Embodiment 18
[0149] Example 18 Synthesis of Compound 18
[0150] The preparation method is as follows:
[0151] Step a. Synthesis of (E)-N-(4-(piperidin-4-yl)butyl)-3-(3-pyridyl)acrylamide:
[0152] The compound 4-(4-aminobutyl) piperidine-1-carboxylic acid tert-butyl ester (2.56g, 10.0mmol) and (E)-3-(3-pyridine) acrylic acid (1.49g, 10.0mmol), HATU (5.70g, 15.0mmol), DIPEA (1.90g, 15.0mmol) were dissolved in dry DMF (20mL), reacted at room temperature for 2h, the reaction solution was poured into 200mL saturated sodium chloride solution, and extracted with ethyl acetate (30mL×3), the organic phases were combined and then washed with saturated brine 30mL to remove the remaining DMF, dried over anhydrous sodium sulfate and concentrated, and purified by silica gel column chromatography (eluent, dichloromethane / methanol=100 / 4) , Get 3.10g of white solid, the obtained product is dissolved in dry CH 2 Cl 2 TFA (6 mL) was added to (30 mL), and reacted overnight at room temperature. The reaction solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com