Development and application of tumor treatment agent containing antibody
An antibody, bispecific antibody technology, applied in anti-tumor drugs, applications, antibodies, etc., can solve problems such as deletion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Embodiment 1, the preparation of B7-H3 recombinant protein
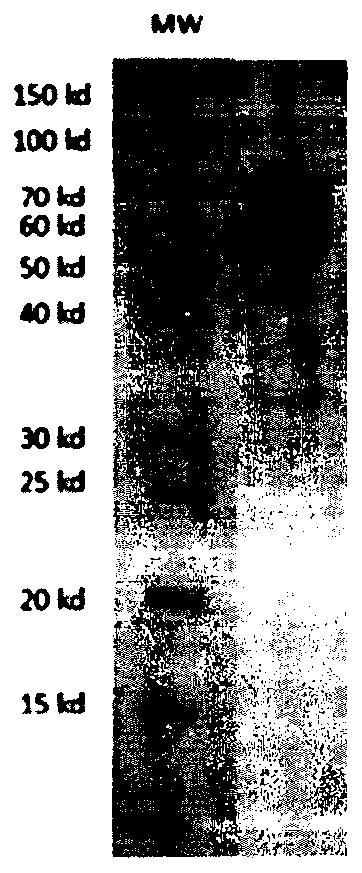
[0155] Using the plasmid pMD18-B7-H3 (Sino Biological Inc.) containing the cDNA sequence of the human B7-H3 gene (SEQ ID NO: 1) as a template, using the forward primer 5'-CTGAGAGGTGCCAGATGTATGCTGCGTCGGCGGGGC-3', the reverse primer 5'-TCCGCCTCCGCCGCTAGCTGTCATAGGCTGCCCTGTG-3 ', the human B7-H3 extracellular region fragment (Met1-Thr461) was amplified by PCR, and the amplified fragment was digested with BspQI and then cloned into the self-constructed eukaryotic expression plasmid system (pSect). HEK293E cells were transfected with this plasmid, and after 6 days, the culture supernatant was collected and purified by nickel column affinity chromatography to obtain the recombinant protein of human B7-H3 extracellular region (SEQ ID NO: 2, the results are as follows: figure 1 shown).
Embodiment 2
[0156] Example 2, cell lines expressing the full length of human B7-H3 or the IgC domain
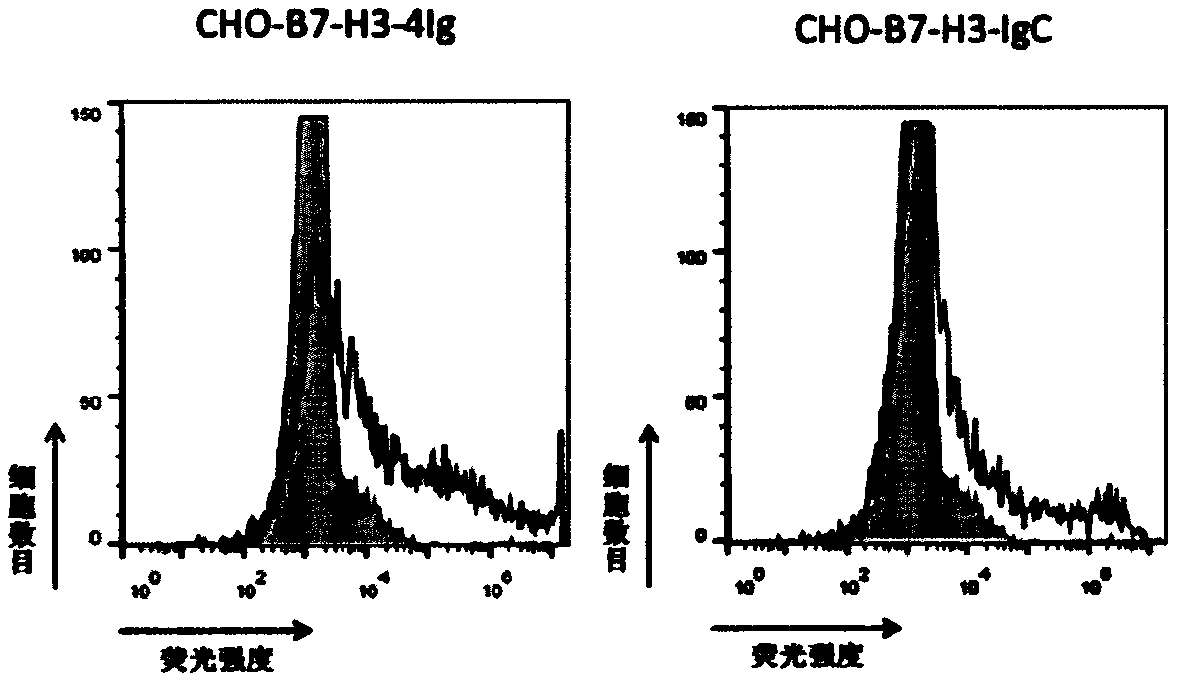
[0157] B7-H3 is widely and highly expressed in tumor cell lines, and the common cell line HEK293 cells also highly express B7-H3 (ShiJ, 2016). Therefore, in the preliminary screening, B7-H3 recombinant protein and HEK293 cells can be used alternately. In order to further confirm the binding specificity of the candidate antibody, the full-length B7-H3 (B7-H3-4Ig) or IgC (B7-H3-IgC) was constructed as a eukaryotic cell expression plasmid and transiently transfected into CHO cells.
[0158] Spread CHO-k1 cells into a 10cm cell culture dish 16-24 hours in advance, make the confluence reach about 90% during transfection, collect 5×10 6 cells, washed 3 times with PBS and resuspended in Opti-MEM medium, the voltage of the electroporation instrument was set to 300V, and the capacitance was 950μF, and the constructed human B7-H3 full-length sequence (SEQ ID NO: 1), B7-H3 IgC sequence The eukaryo...
Embodiment 3
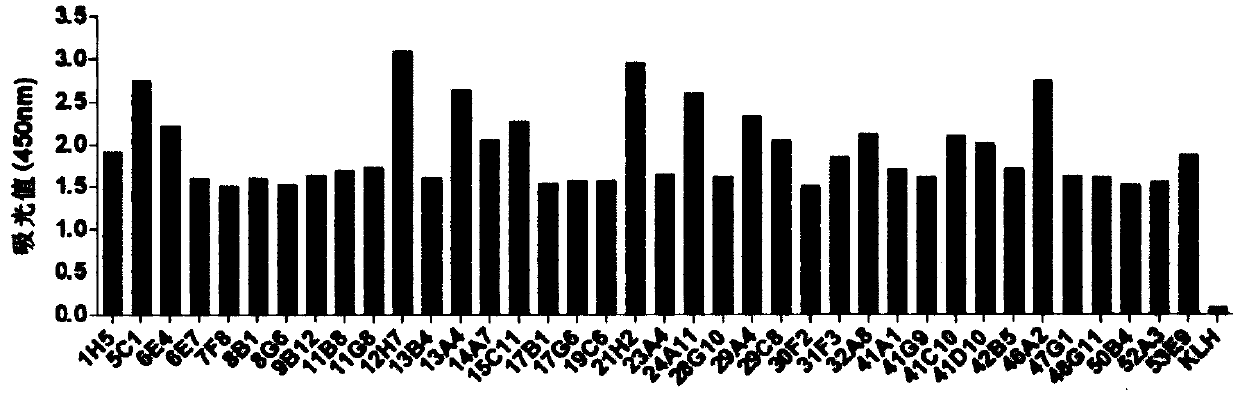
[0159] Embodiment 3, preparation of anti-human B7-H3 monoclonal antibody
[0160] 1) Immune mice
[0161] Mix 400 μL human B7-H3 recombinant protein with a concentration of 1 mg / mL as an antigen with an equal volume of immune adjuvant (Titermax adjuvant, Sigma-Aldrich), and take six 6-week-old female Balb / c mice (Beijing Weitongli Hua) for subcutaneous immunization. After the primary immunization, a booster immunization of the same dose was given every two weeks.
[0162] 2) Cell fusion and hybridoma screening
[0163] Three days before fusion, 10 μg / 100 μL / human B7-H3 recombinant protein was used for tail vein pulse immunization. Three days later, the spleen and lymph nodes of the mice were taken and ground in DMEM to obtain a B cell suspension. After mixing an appropriate amount of the B cell suspension with SP2 / 0, the cells were fused using an electrofusion apparatus. The fused cells were placed in DMEM complete medium containing HAT in 5% CO 2 , cultured at 37°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com