Application of zingerenone a in the preparation of drugs for preventing and treating colitis
A technology for shogatonone and colitis, which is applied in the application field of shogatonone A in preventing and treating colitis, and achieves the effects of reducing colon atrophy, inhibiting colonic mucosal edema, and enhancing intestinal barrier function.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
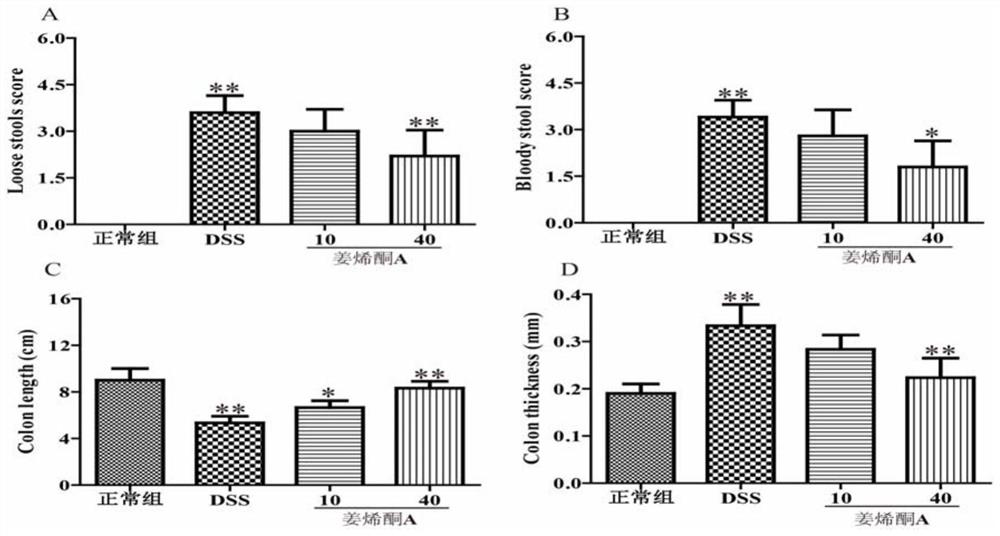
[0022] Embodiment 1 Zingerenone A affects the clinical symptoms and colon length and thickness of ulcerative colitis model
[0023] 1. Experimental animals:
[0024] SPF grade, male BALB / c mice (6-8 weeks old, 18-22g), purchased from the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine, license number: SCXK (Guangdong) 2013-0034, animal certificate number: NO .44007200043643. The animals were kept in the SPF experimental animal room of the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine. The animal feeding conditions were: temperature (20-25°C), humidity (65-70%), 12h light and dark alternately, and the animals were given standard feed during feeding. and sterile distilled water ad libitum, and new litter every other day.
[0025] 2. Experimental method:
[0026] Male BALB / C mice (6-8 weeks old, 18-22g), after 7 days of adaptive feeding, the animals were randomly divided into: normal control group (Normal ...
Embodiment 2
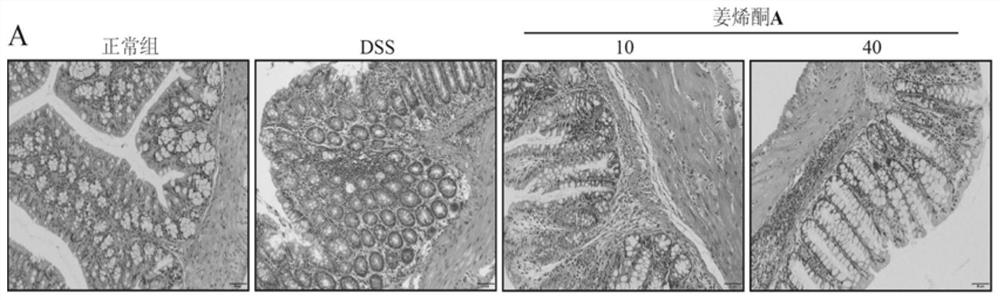
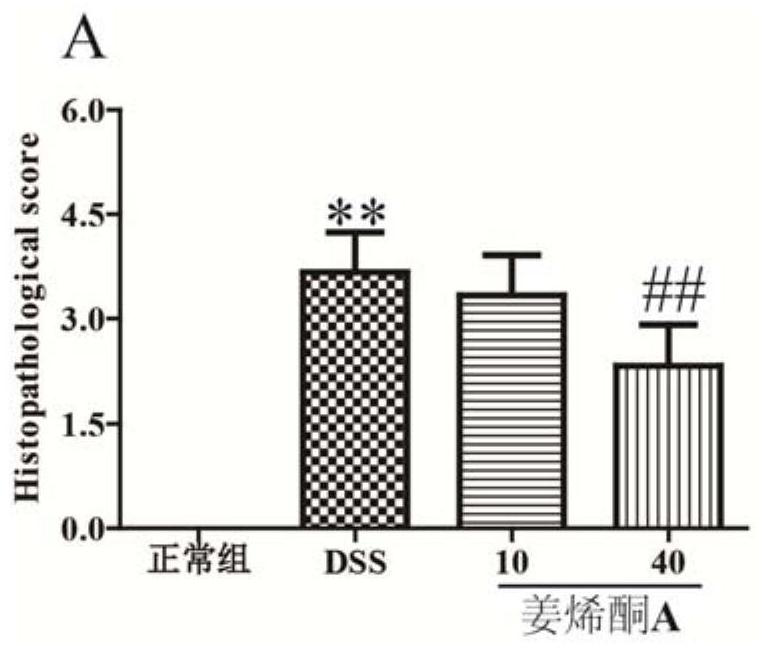
[0030] Example 2 Effect of zingerenone A on colon histopathology in animal model of ulcerative colitis
[0031] The excavated colon tissue after the experiment of Example 1 was taken for histopathological examination of the colon: the colon tissue was fixed in 4% paraformaldehyde fixative. After soaking for 24 hours, the colon tissues were dehydrated and embedded in wax blocks. The thickness of the slices was 4 μm, and the slices were placed in an automatic stainer for HE staining. After HE staining, the slides were mounted with neutral resin. The morphology and inflammatory infiltration of the tissue were observed under a light microscope. The result is as figure 2 shown.
[0032] Depend on figure 2 It can be seen that the structure and morphology of the colon tissue of animals in the DSS model group changed significantly, a large number of inflammatory cells infiltrated, goblet cell necrosis and loss were serious, goblet cell swelling was obvious, the colonic mucosal ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com