A kind of preparation method of mivacurium chloride and injection thereof
A technology of mivacurium chloride and injection, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as stability and safety issues that have not been properly resolved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
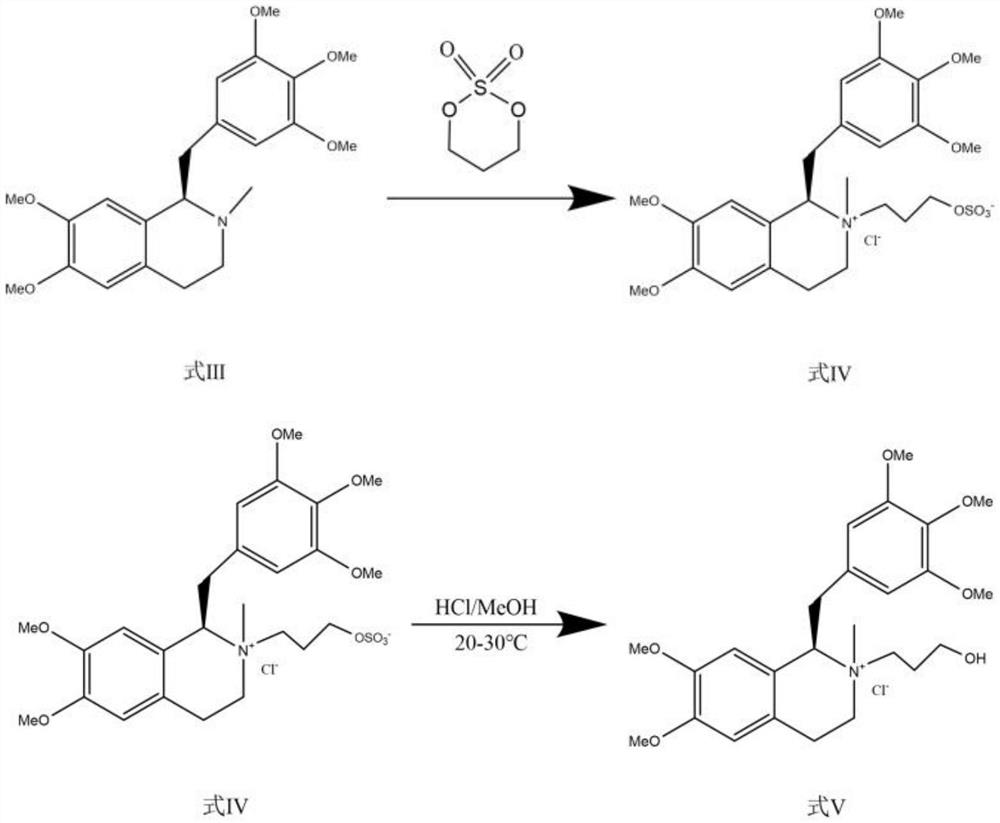
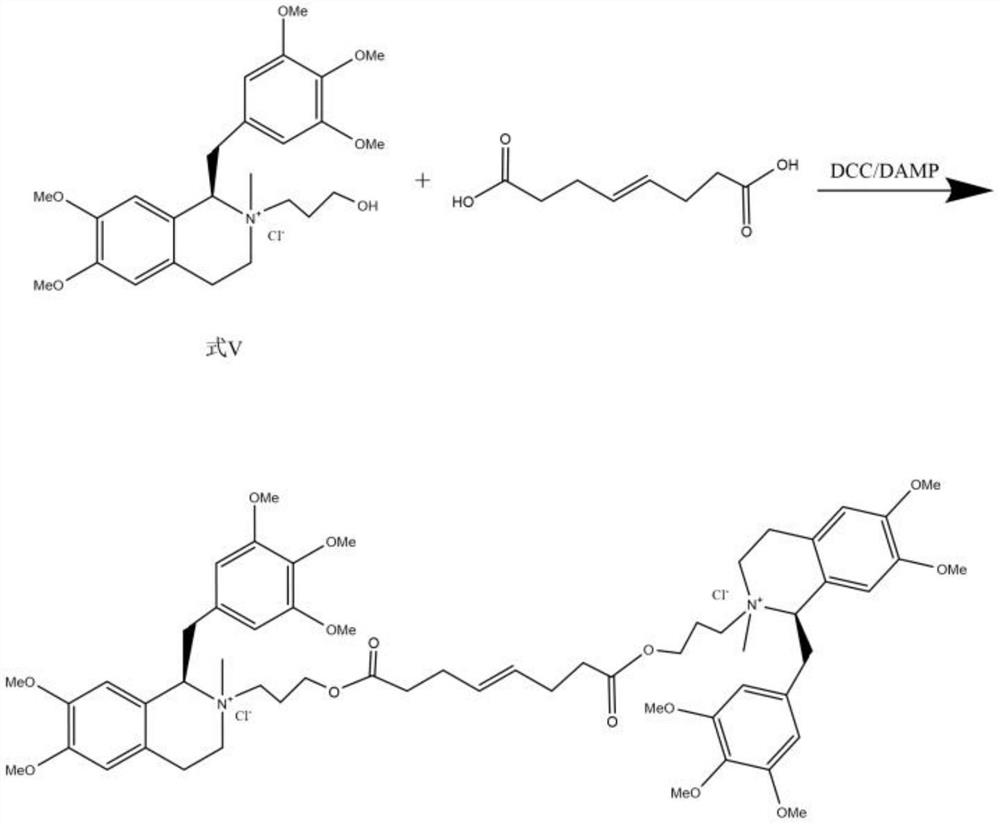
[0052] A kind of synthetic method of mivacurium chloride, comprises the steps:
[0053] Step 1: Asymmetric Catalytic Reduction
[0054]
[0055] The crude product (100g) of the compound of formula VI was dissolved in tetrahydrofuran (1000ml), ultrasonically degassed, replaced with nitrogen for 5 minutes, added metal chiral catalyst RuCl-Ts-DPEN (2500mg), formic acid-triethylamine (V / V= 5:3, 15 mL), reacted at 15°C for 2.5 hours. Add saturated NaHCO 3 Aqueous solution (3000mL) was quenched, extracted continuously 3 times with ethyl acetate (2000ml), combined extracts; washed extracts 2 times with saturated brine (500ml) and dried, concentrated dry extract The R form of the compound of formula I (91.9 g) was obtained.
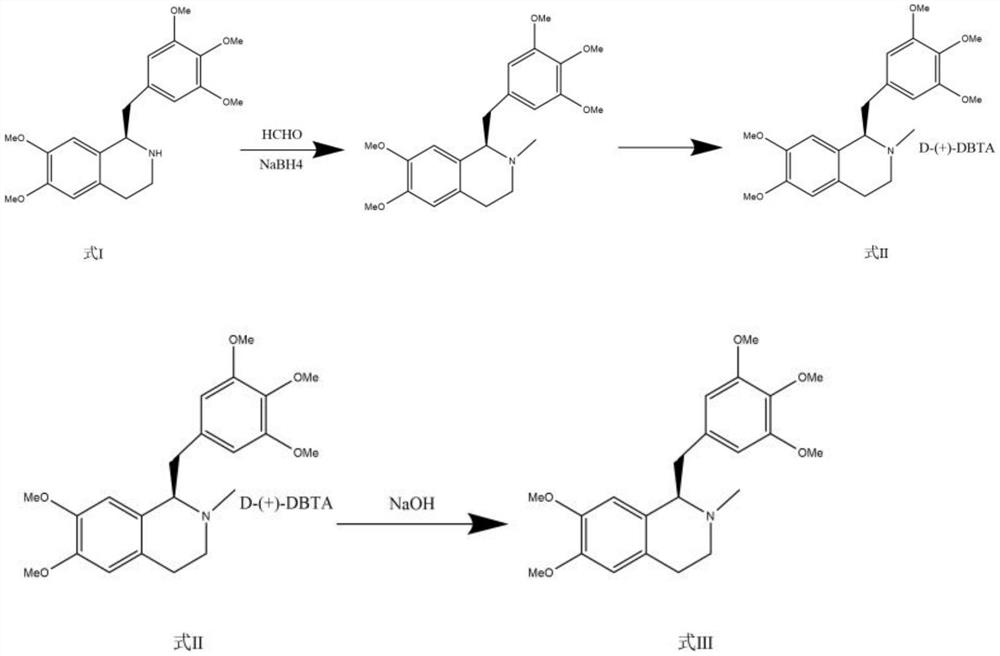
[0056] Step 2: Methylation and purification of amines
[0057]
[0058] Add the compound of formula I (100 g) and sodium borohydride (120 g) into 1500 ml of ethyl acetate, then add 1200 ml of 37% formaldehyde solution, and reflux until the reaction is c...
Embodiment 2
[0067] A kind of synthetic method of mivacurium chloride, comprises the steps:
[0068] Step 1: Asymmetric Catalytic Reduction
[0069] The crude product (100g) of the compound of formula VI was dissolved in dichloromethane (1000ml), ultrasonically degassed, replaced with nitrogen for 5 minutes, added metal chiral catalyst RuCl-Ts-DPEN (2200mg), formic acid-triethylamine (V / V=9:4, 20 mL), reacted at 0°C for 7.5 hours. Add saturated NaHCO 3 Aqueous solution (3000mL) was quenched, extracted continuously 3 times with dichloromethane (2000ml), combined extract; After washing extract with saturated brine (500ml) 2 times, dried, concentrated dry extract obtained the formula I compound of R type ( 90.3g).
[0070] Step 2: Methylation and purification of amines
[0071] Add the compound of formula I (100 g) and sodium borohydride (95 g) into 1500 ml of tetrahydrofuran, and then add 1400 ml of 30% formaldehyde solution, and reflux until the reaction is complete. After cooling to ...
Embodiment 3
[0077] A kind of synthetic method of mivacurium chloride, comprises the steps:
[0078] Step 1: Asymmetric Catalytic Reduction
[0079]The crude product (100g) of the compound of formula VI was dissolved in methyl tert-butyl ether (1000ml), ultrasonically degassed, replaced with argon for 5 minutes, and added metal chiral catalyst RuCl-Ts-DPEN (2000mg), formic acid-triethyl Amine (V / V=3:2, 18 mL), react at 4°C for 6.5 hours. Add saturated NaHCO 3 Quench with aqueous solution (2500mL), extract continuously with methyl tert-butyl ether (2000ml) for 3 times, combine the extracts; wash the extract with saturated brine (500ml) for 2 times and dry, then concentrate and dry the extract to obtain the R-type formula Compound I (91.1 g).
[0080] Step 2: Methylation and purification of amines
[0081] Add the compound of formula I (100 g) and sodium borohydride (90 g) into 1500 ml of dichloromethane, then add 800 ml of 40% formaldehyde solution, and reflux until the reaction is comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com