Method for detecting crizotinib intermediate
A technology of crizotinib and detection method, which is applied in the field of analysis, can solve the problems of not finding crizotinib intermediates, etc., and achieve the effect of improving separation effect, high applicability, and improving accuracy and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] This embodiment provides a detection method for crizotinib intermediates, comprising the following steps:
[0085] (1) Dissolve the racemate of crizotinib intermediate 1 in an organic solvent to obtain a control sample solution A1; dissolve the crizotinib intermediate 1 to be tested in an organic solvent to obtain a test sample solution B1 ;
[0086] The organic solvent is formed by mixing n-hexane, isopropanol and diethylamine in a volume ratio of 50:50:0.5. The concentration of control sample solution A1 and test sample solution B1 is 0.5mg / mL.
[0087] (2) Get described control sample solution A1, inject liquid chromatograph, record the chromatogram of control sample A1.
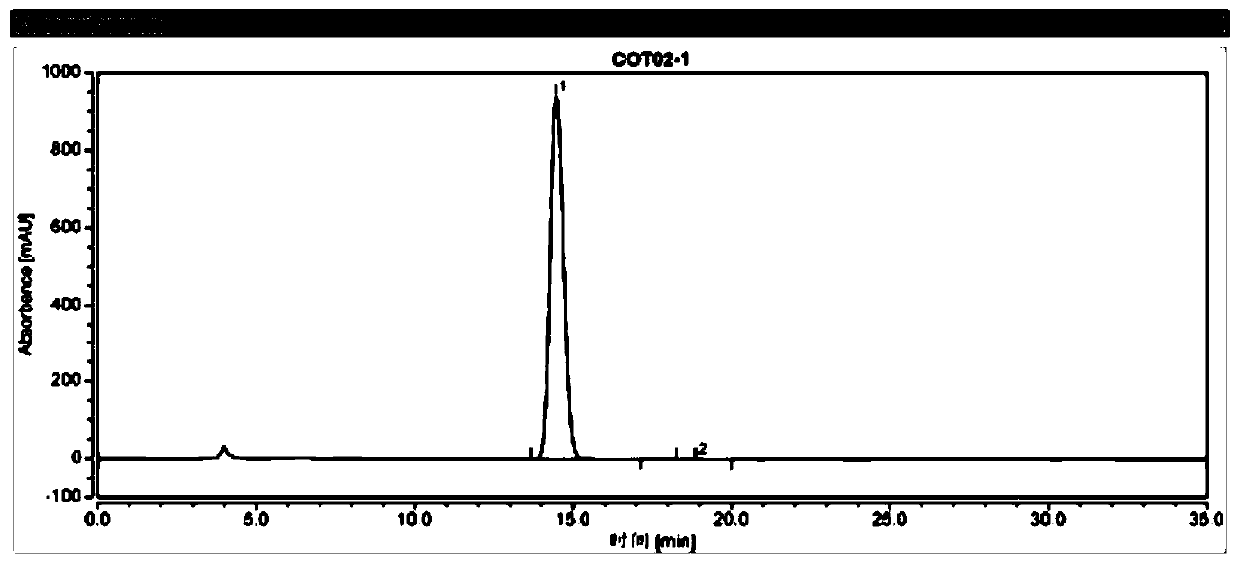
[0088] (3) get described test sample solution B1, inject liquid chromatograph, record the chromatogram of test sample B1, calculate the percentage of enantiomer by area normalization method, the results are shown in Table 1 and figure 1 .
[0089] The chromatographic conditions in the present emb...
Embodiment 2
[0103] This embodiment provides a detection method for crizotinib intermediates, comprising the following steps:
[0104] (1) control sample solution is the same as embodiment 1; Crizotinib intermediate 2 to be tested is dissolved in an organic solvent to obtain test sample solution B2;
[0105] The organic solvent is formed by mixing n-hexane, isopropanol and diethylamine in a volume ratio of 50:50:0.5. The concentration of control sample solution A1 and test sample solution B2 is 0.5mg / mL.
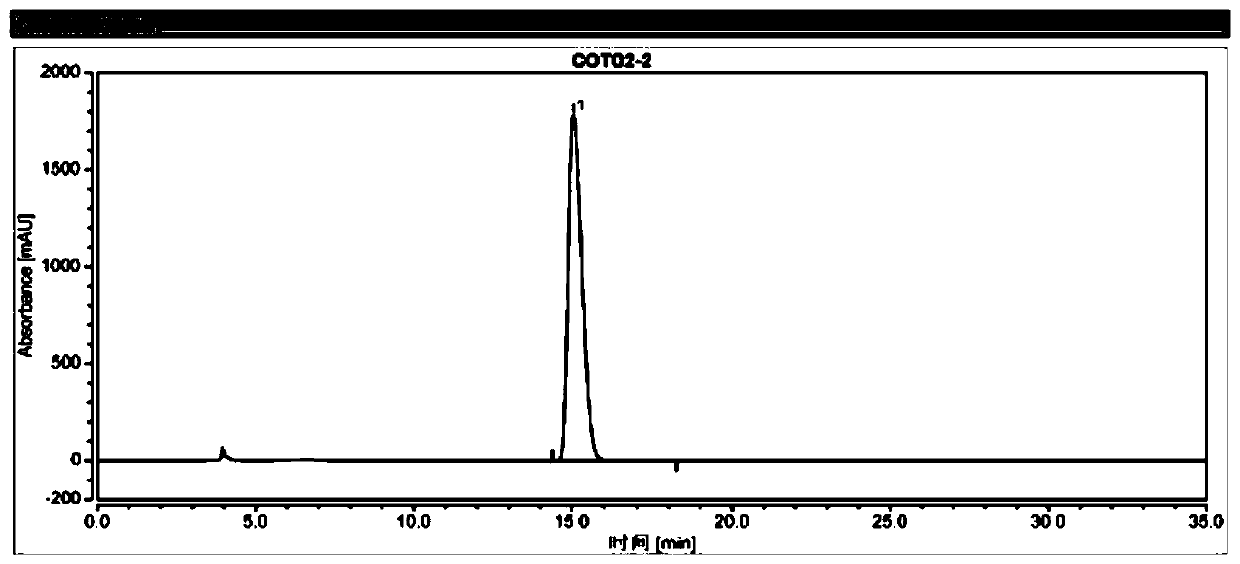
[0106] (2) get described test sample solution B2, inject liquid chromatograph, record the chromatogram of test sample B2, calculate the percentage of enantiomer by area normalization method, the results are shown in Table 2 and figure 2 .
[0107] The chromatographic conditions in the present embodiment are:
[0108] Chromatographic column: chiralcel AD-H (4.6*250mm, 5μm);
[0109] Injection volume: 20μL;
[0110] Flow rate: 1.0mL / min;
[0111] Column temperature: room temperature...
Embodiment 3
[0120] This embodiment provides a detection method for crizotinib intermediates, comprising the following steps:
[0121] (1) control sample solution is the same as embodiment 1; Crizotinib intermediate 3 to be tested is dissolved in an organic solvent to obtain test sample solution B3;
[0122] The organic solvent is formed by mixing n-hexane, isopropanol and diethylamine in a volume ratio of 50:50:0.5. The concentration of control sample solution A1 and test sample solution B3 is 0.5mg / mL.
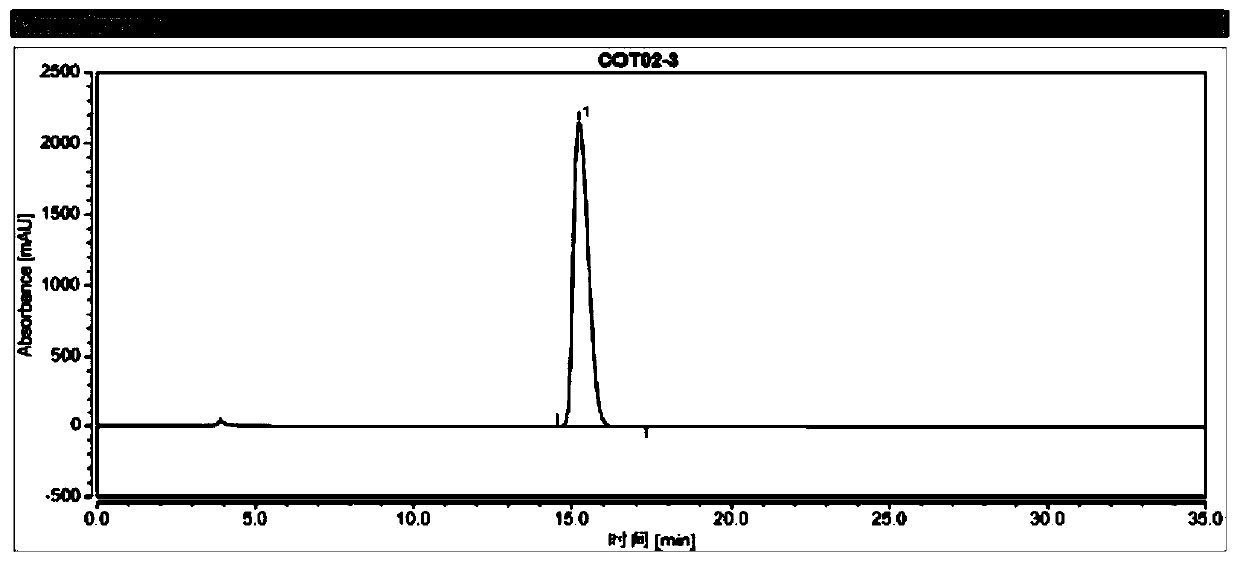
[0123] (2) get described test sample solution B3, inject liquid chromatograph, record the chromatogram of test sample, calculate the percentage of enantiomer by area normalization method, the results are shown in Table 3 and image 3 .
[0124] The chromatographic conditions in the present embodiment are:
[0125] Chromatographic column: chiralcel AD-H (4.6*250mm, 5μm);
[0126] Injection volume: 20μL;
[0127] Flow rate: 1.0mL / min;
[0128] Column temperature: room temperature;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com