Curable resin composition comprising phthalonitrile oligomers, and prepolymer thereof
A phthalonitrile, curing resin technology, used in applications, inks, household appliances, etc., can solve the problems of low viscosity, mold leakage, etc., and achieve the effect of excellent wettability and excellent processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
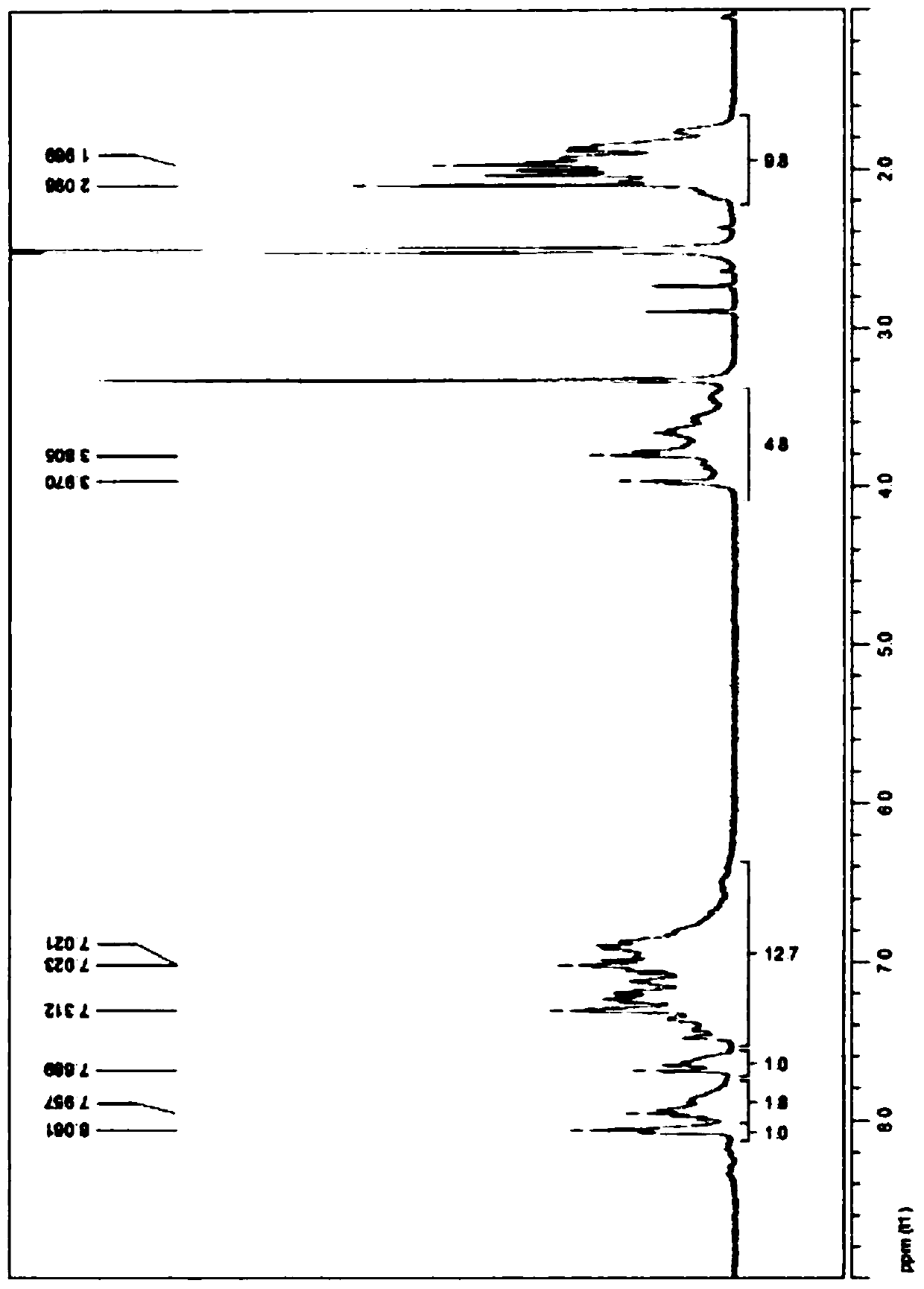
[0237] Preparation example 1. Synthesis of phthalonitrile oligomer (PN-1)
[0238] A phthalonitrile oligomer represented by the following chemical formula PN-1 was synthesized as follows.
[0239]
[0240] 41.1 g of an oligomer represented by chemical formula KA-1 (weight average molecular weight 1,360 g / mol) and 165.0 g of DMF (dimethylformamide) were introduced into a 3-necked round bottom flask, and the mixture was stirred at room temperature to dissolve . 51.9 g of 4-nitrophthalonitrile was added thereto, 70.0 g of DMF was added, and the mixture was stirred to dissolve. Subsequently, 49.8 g of potassium carbonate and 30.0 g of DMF were introduced together, and the temperature was raised to 85° C. while stirring. After allowing the reaction to proceed for about 5 hours, the reaction solution was cooled to room temperature. The cooled reaction solution was poured into 0.2N aqueous hydrochloric acid solution for neutralization and precipitation. After filtering, it w...
preparation example 2
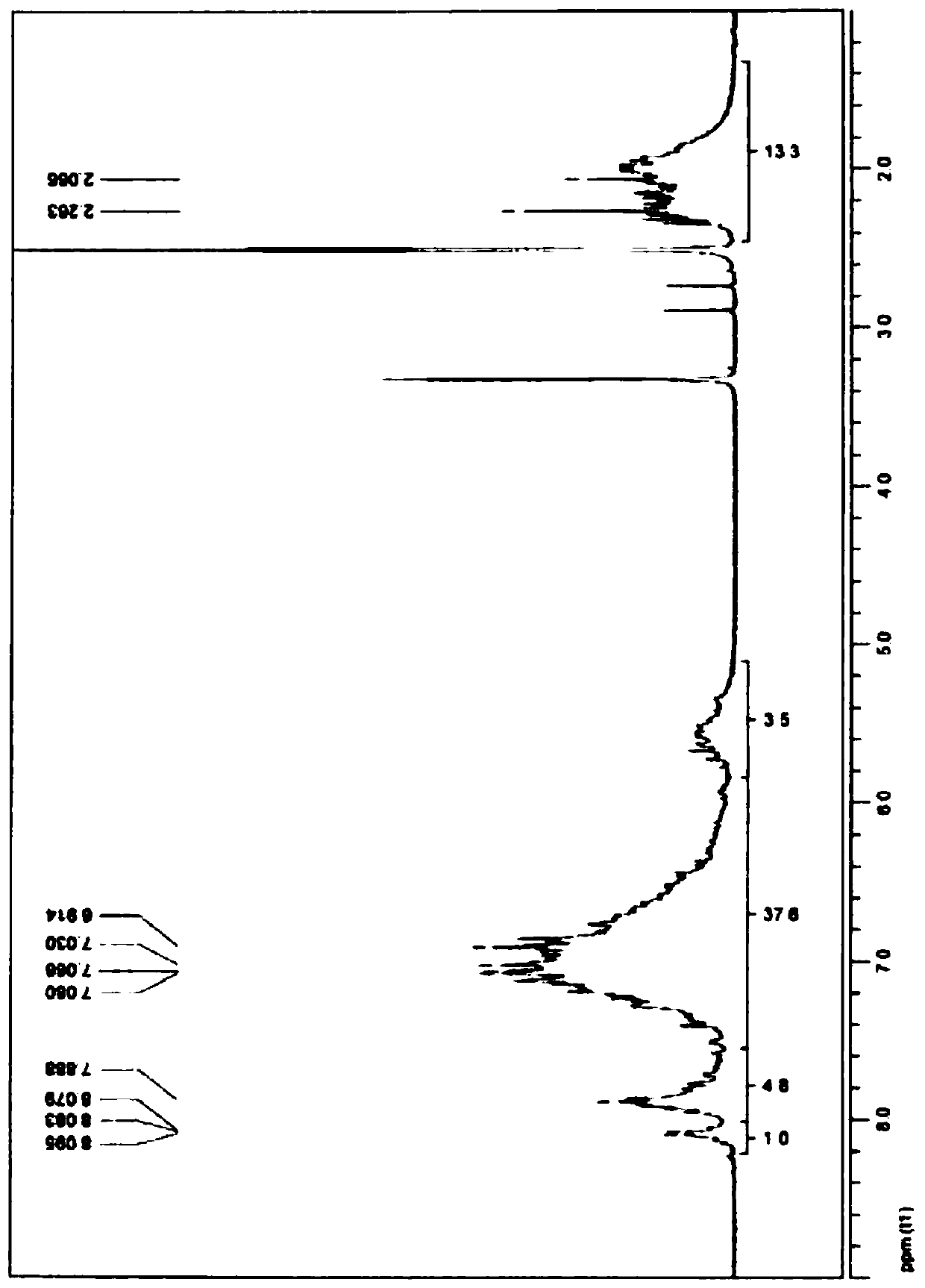
[0242] Preparation example 2. Synthesis of phthalonitrile oligomer (PN-2)
[0243] A phthalonitrile oligomer represented by the following chemical formula PN-2 was synthesized as follows.
[0244]
[0245] 48.0 g of an oligomer represented by chemical formula NE-1 (weight average molecular weight 1,600 g / mol) and 165.0 g of DMF (dimethylformamide) were introduced into a 3-necked round bottom flask, and the mixture was stirred at room temperature to dissolve . 48.5 g of 4-nitrophthalonitrile was added thereto, 70.0 g of DMF was added, and the mixture was stirred to dissolve. Subsequently, 46.4 g of potassium carbonate and 30.0 g of DMF were introduced together, and the temperature was raised to 85° C. while stirring. After allowing the reaction to proceed for about 5 hours, the reaction solution was cooled to room temperature. The cooled reaction solution was poured into 0.2N aqueous hydrochloric acid solution for neutralization and precipitation. After filtering, it w...
preparation example 3
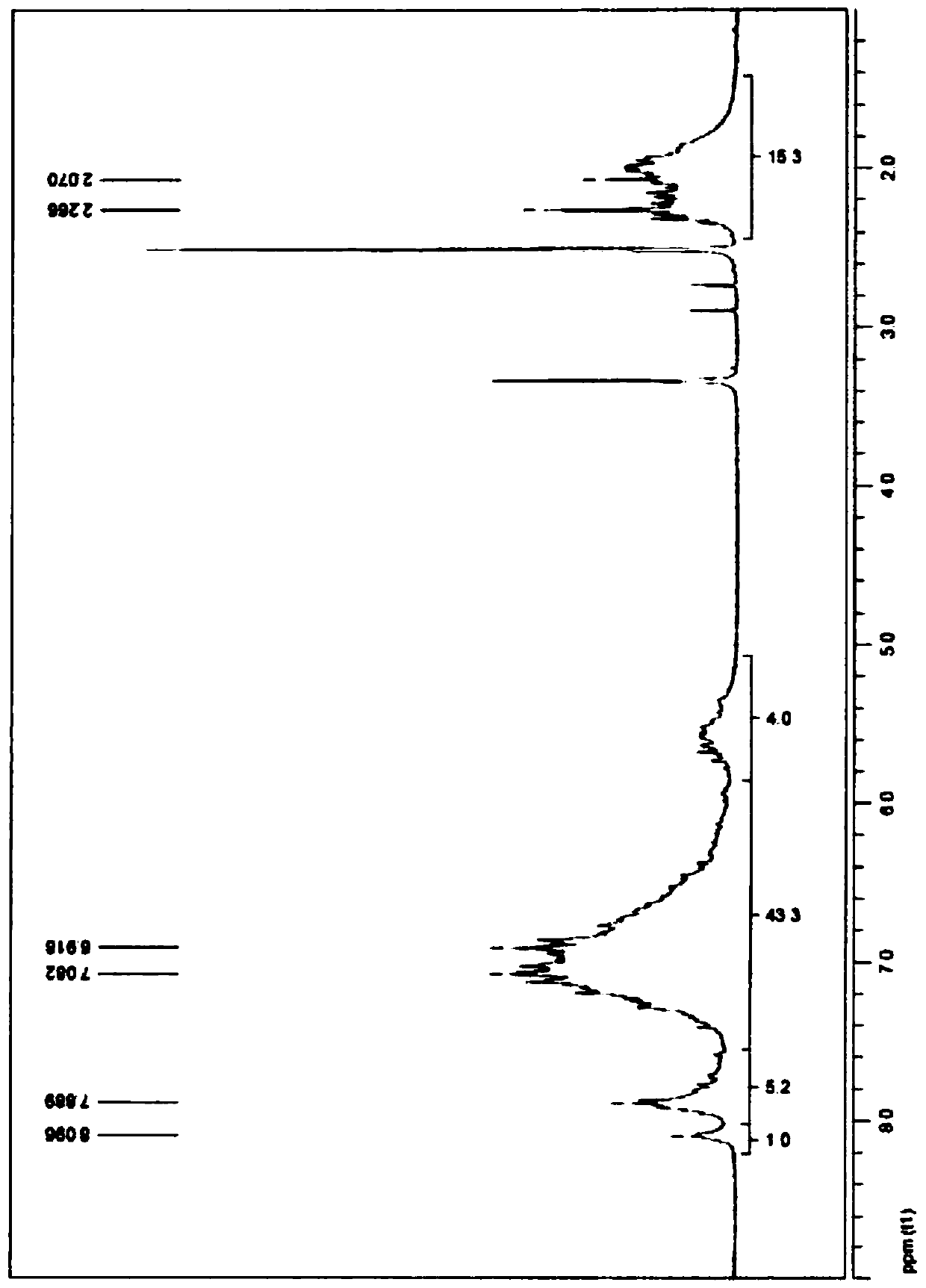
[0247] Preparation Example 3: Synthesis of phthalonitrile oligomer (PN-3)
[0248] The oligomer (PN-3) represented by the chemical formula PN-2 having a weight-average molecular weight of 2,400 g / mol was obtained in a yield of 96% by weight by the same method as Preparation Example 2 except that 1,700 g The oligomer represented by the chemical formula NE-1 of the weight average molecular weight per mol was substituted for the oligomer represented by the chemical formula NE-1 (weight average molecular weight 1,600 g / mol).
[0249]
[0250] image 3 shows the phthalonitrile oligomer PN-3 1 H-NMR analysis results.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com