The preparation method of 4-amino-2-cyclopentene-1-methanol hydrochloride
A technology of hydrochloride and cyclopentene, applied in the field of preparation of 4-amino-2-cyclopentene-1-methanol hydrochloride, can solve the problems of high pollution, high cost, and low price of reaction raw materials, and achieve Low price, avoid contact and reaction, reduce cost and pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
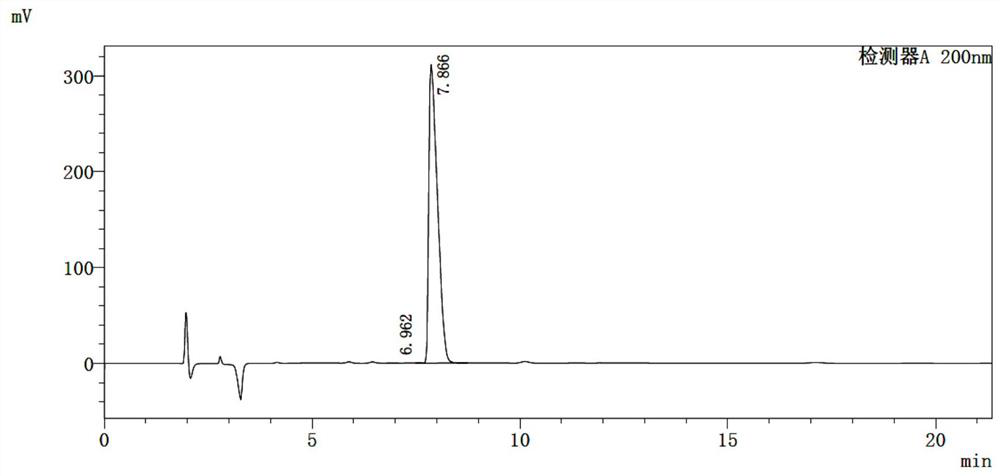
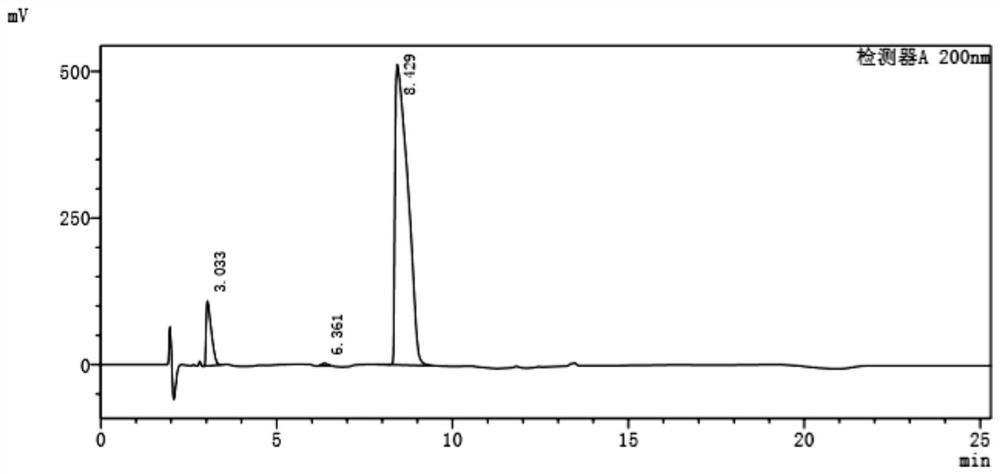
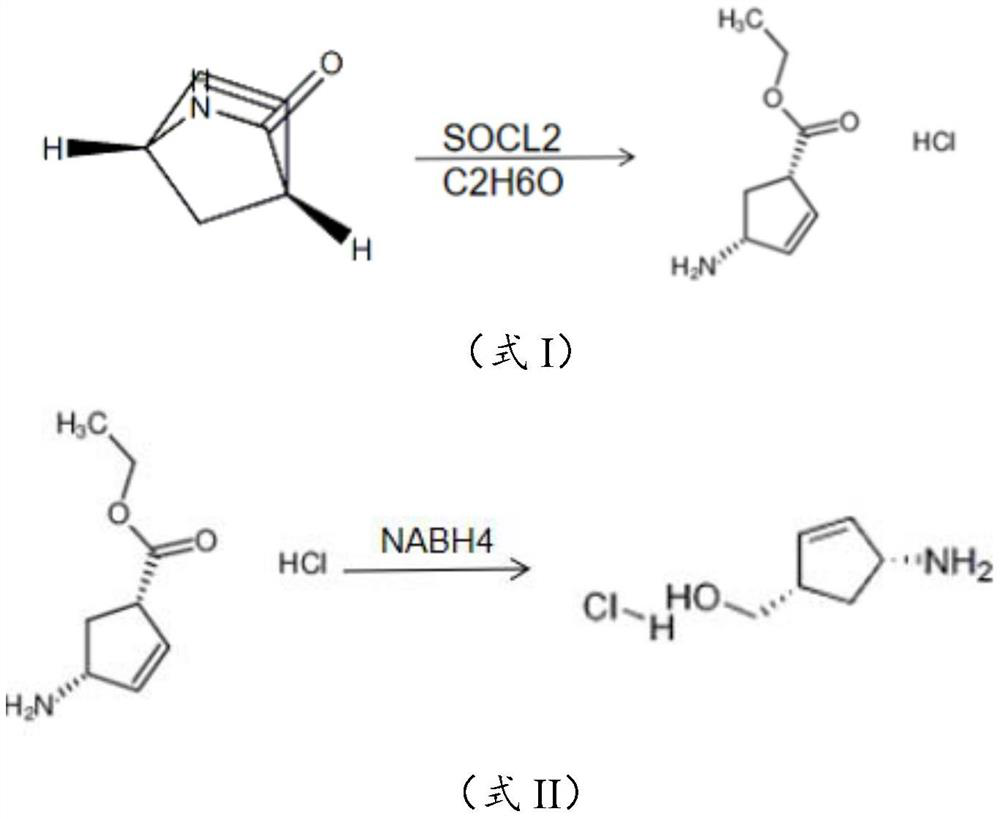
[0016] The embodiment of the present application provides a preparation method of 4-amino-2-cyclopentene-1-methanol hydrochloride, comprising: 2-azabicyclo[2,2,1]hept-5-ene-3- The ketone undergoes esterification and ring-opening reaction in the presence of thionyl chloride to obtain the ring-opening compound hydrochloride; the ring-opening compound hydrochloride is subjected to reduction treatment in an aqueous solution of dichloromethane to obtain 4-amino-2 - A reaction solution of cyclopentene-1-methanol hydrochloride.
[0017] In the examples of the present application, 2-azabicyclo[2,2,1]hept-5-en-3-one was esterified and ring-opened in the presence of thionyl chloride to obtain A structurally stable ring-opened compound that exists in the form of a salt. The ring-opening compound hydrochloride is reduced in an aqueous solution of dichloromethane. During the reduction process, the organic amine is effectively dissociated into the dichloromethane and the ester group is dis...
Embodiment 1
[0038] A preparation method of (1S,4R)-4-amino-2-cyclopentene-1-methanol hydrochloride, comprising:
[0039] S1. Add 50g of (1R,4S)-2-azabicyclo[2,2,1]hept-5-en-3-one and 100g of absolute ethanol in the flask, and cool down to 0°C while stirring. Then, 60 g of thionyl chloride was added dropwise to the system, and the temperature of the system was controlled to be less than 10° C. during the dropwise addition. After the addition of thionyl chloride was completed, the system was heated to 30°C and stirred for 1 hour. After the reaction, the (1S,4R)-(-)ethyl-4-aminocyclopent-2-ene-1-carboxylate obtained The salt hydrochloride is concentrated and dried for later use.
[0040] S2. (1S,4R)-(-)ethyl-4-aminocyclopent-2-ene-1-carboxylate hydrochloride obtained in S1 was dissolved in 200mL water and 200mL dichloromethane mixed to obtain the dichloromethane In the aqueous solution of methyl chloride, stir to dissolve and clarify and cool down to below 10°C to obtain a mixed solution; ...
Embodiment 2
[0043] A preparation method of (1S,4R)-4-amino-2-cyclopentene-1-methanol hydrochloride, comprising:
[0044] S1. Add 50g of (1R,4S)-2-azabicyclo[2,2,1]hept-5-en-3-one and 100g of absolute ethanol in the flask, and cool down to 0°C while stirring. Then, 60 g of thionyl chloride was added dropwise to the system, and the temperature of the system was controlled to be less than 10° C. during the dropwise addition. After the addition of thionyl chloride was completed, the system was heated to 30°C and stirred for 1 hour. After the reaction, the (1S,4R)-(-)ethyl-4-aminocyclopent-2-ene-1-carboxylate obtained The salt hydrochloride is concentrated and dried for later use.
[0045] S2. (1S,4R)-(-)ethyl-4-aminocyclopent-2-ene-1-carboxylate hydrochloride obtained in S1 was dissolved in 175mL water and 205mL dichloromethane mixed to obtain dichloromethane In the aqueous solution of methyl chloride, stir to dissolve and clarify and cool down to below 10°C to obtain a mixed solution; add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com