Application of Letemovir in preparation of SARS-CoV-2 novel coronavirus inhibitor
A sars-cov-2, coronavirus technology, applied in the field of medicine, can solve problems such as unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
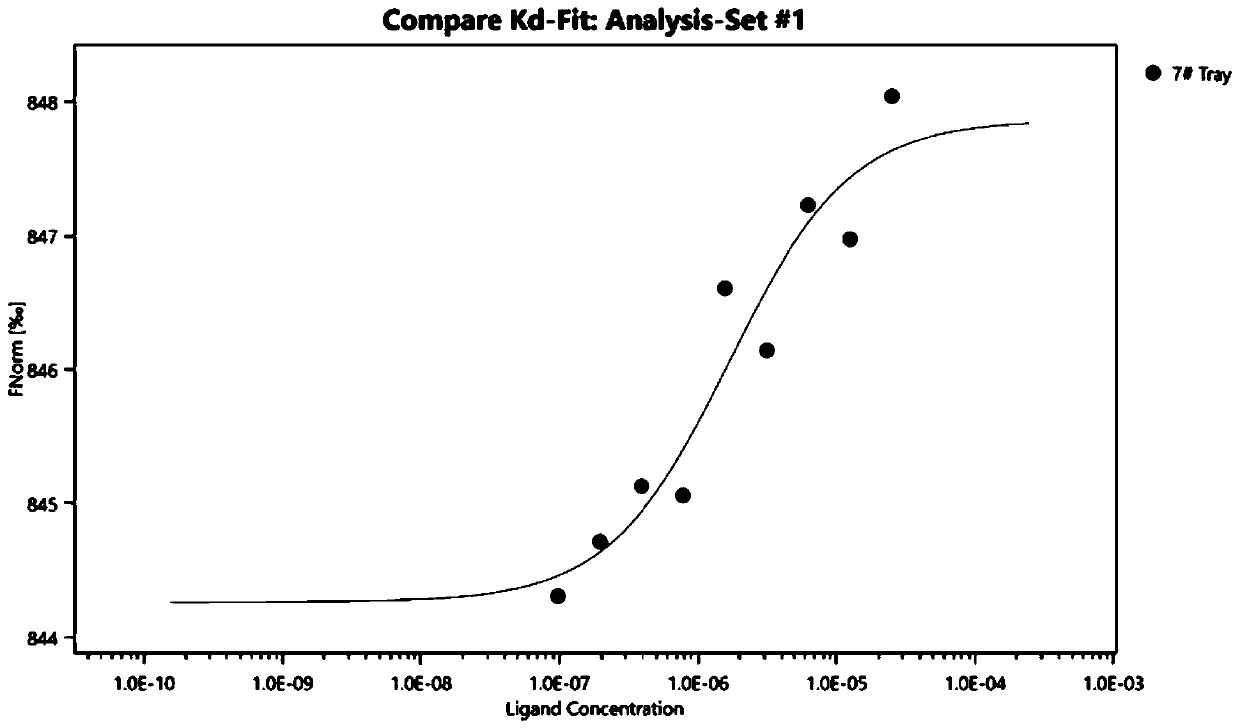
[0038] Embodiment 1 Microscale Thermophoresis (MicroscaleThermophoresis, MST) measures the direct binding of Letermovir to the SARS-CoV-2 novel coronavirus S protein RBD region
[0039] 1. Principle
[0040] This technology is based on the thermophoretic phenomenon of biomolecules. MST uses infrared lasers for local heating to cause directional movement of molecules, and then analyzes the directional movement of particles in microscopic temperature gradients through fluorescence. Changes in the size, charge and hydration layer of biomolecules due to binding can be detected.
[0041] 2. Application
[0042] (1) The binding process of the single-chain antibody to the human antigen protein;
[0043] (2) The interaction between small molecule compounds and model proteins;
[0044] (3) Interaction between in vitro synthesized protein and conjugate molecule;
[0045] (4) The itinerary of the three-dimensional helical structure of DNA / RNA molecules;
[0046] (5) The inhibitory e...
Embodiment 2
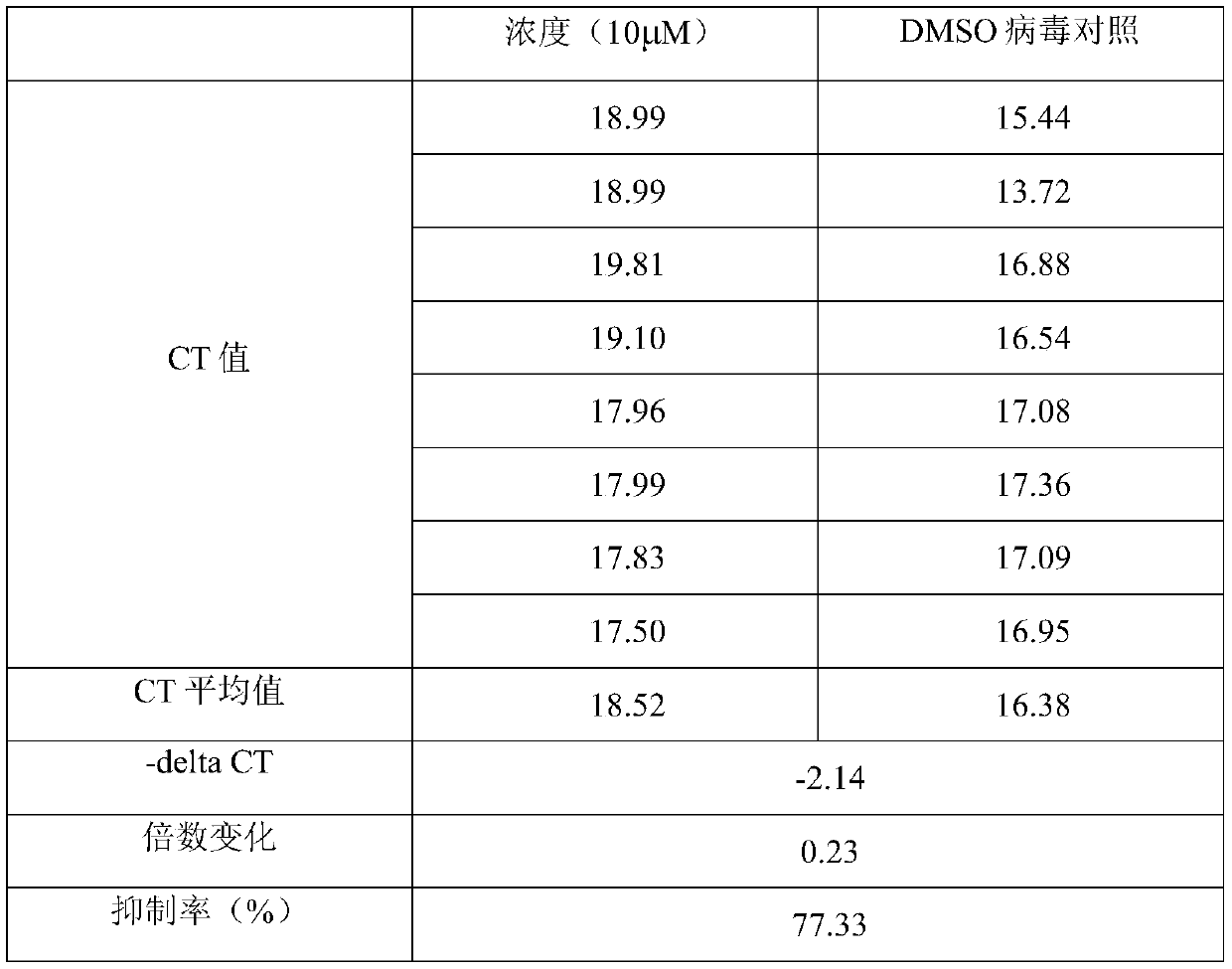
[0054] Example 2 Letermovir anti-SARS-CoV-2 activity effect in vitro
[0055] 1. Biosafety requirements
[0056] The in vitro anti-SARS-CoV-2 activity evaluation experiment of Letermovir involves virus culture. According to the requirements of the National Health and Medical Commission's "Biosafety Guidelines for Novel Coronavirus Laboratories (Second Edition)", all virus cultures are conducted at Biosafety Level 3 (BSL- 3) In the laboratory. Use virus culture to extract nucleic acid, and the addition of lysing agent or inactivating agent is carried out under the same level of laboratory and protective conditions as virus culture. Nucleic acid detection and other operations after the live virus is inactivated by a reliable method are carried out in a biosafety level two laboratory (BSL-2).
[0057] 2. Experimental environment
[0058] Room temperature: 22°C, relative humidity: 55%.
[0059] 3. Experimental method
[0060] 1. Experimental grouping
[0061] (1) Letermovir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com