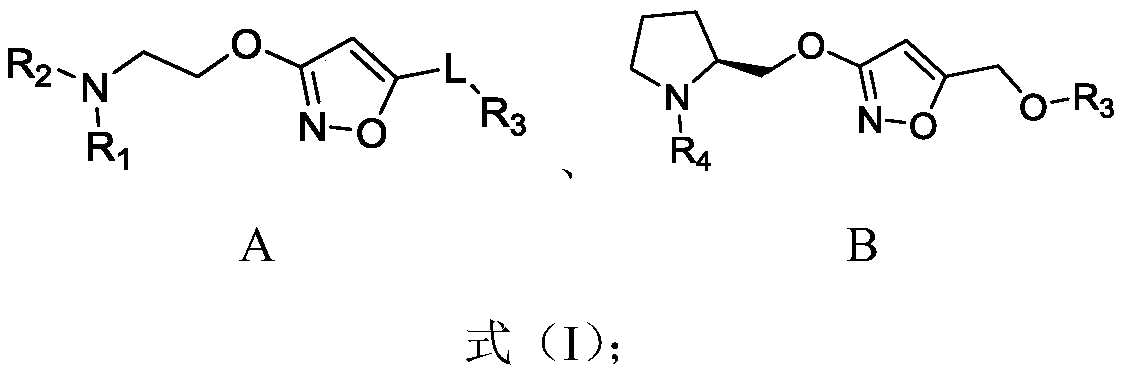
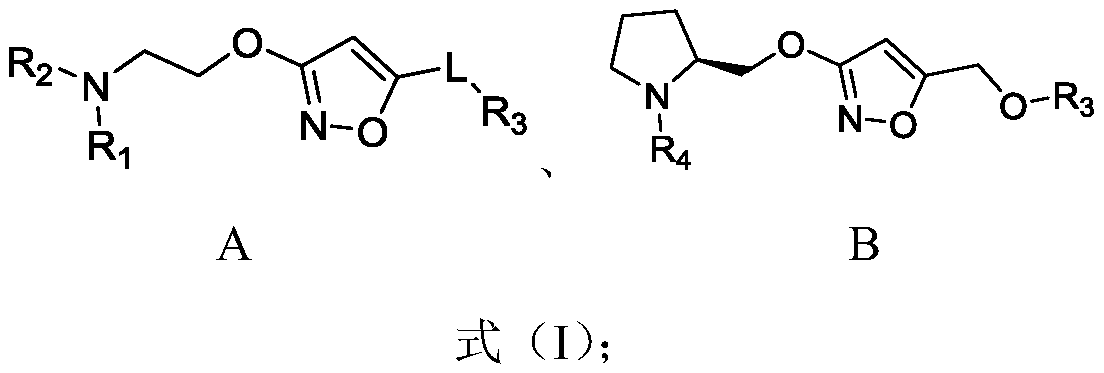
Alkoxy isoxazole derivative and preparation method and application thereof
A technology of alkoxyisoxazole derivatives, which is applied in the field of alkoxyisoxazole derivatives and their preparation and application, can solve the problem of insignificant anti-tumor activity and achieve a good effect of promoting nerve regeneration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
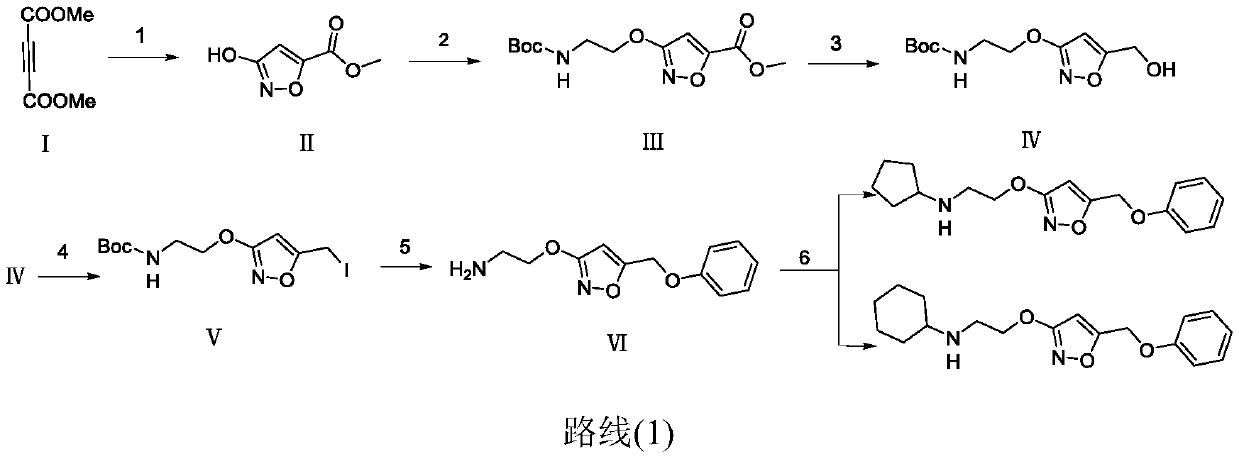
[0106] Embodiment (1) Preparation of 3-(2-(cyclobutyl (methyl) amino) ethoxy)-N-phenylisoxazole-5-carboxamide hydrochloride
[0107] 1.1 Preparation of methyl 3-hydroxyisoxazole-5-carboxylate
[0108] Add compound hydroxyurea (7.6g, 100mmol) and DBU (16.7g, 110mmol) into a 250mL three-necked flask. When the system was cooled to 0°C, 50 mL of solvent methanol was added. The compound butynedioic acid dimethyl was slowly added dropwise under the condition of nitrogen protection. After the dropwise addition, the system was orange-yellow and stirred for 1 h under ice-bath conditions. After 1 h, the reaction was removed from the ice bath, slowly raised to room temperature and stirred overnight. After the reaction, the solvent was spin-dried in vacuo, dissolved in 20 mL of water, and adjusted to pH 1 with hydrochloric acid. The system was extracted with ethyl acetate (3*30mL), anhydrous Na 2 SO 4 dry. The ethyl acetate was spin-dried, and the obtained crude product was recrysta...
Embodiment (8
[0129] Embodiment (8) Preparation of 3-(2-(cyclobutyl(methyl)amino)ethoxy)-N-(3-trifluoromethylphenyl)isoxazole-5-carboxamide hydrochloride
[0130] Add the compound 3-(2-((tert-butoxycarbonyl)(methyl)amino)ethoxy)isoxazole-5-carboxylic acid (350mg, 1.22mmol) prepared in 1.3 to a 100mL round bottom flask, 3 -trifluoromethylaniline (196.6mg, 1.22mmol), EDC (304.8mg, 1.59mmol), HOBt (214.8mg, 1.59mmol) and 3-picoline (0.17mL, 1.71mmol) and 25mLDCM, under nitrogen protection The reaction was stirred overnight at room temperature. After the reaction, the solvent was spin-dried, dissolved in ethyl acetate, washed with water, washed with 1N dilute hydrochloric acid, washed with saturated sodium bicarbonate solution, washed with saturated brine, and dried over anhydrous sodium sulfate. Spin to dry ethyl acetate, after the crude product was obtained by flash column chromatography, add 30mL ethyl acetate hydrochloride and stir overnight, spin to dry ethyl acetate, beat and filter with...
Embodiment (10
[0133] Embodiment (10) Preparation of 3-(2-(cyclobutyl(methyl)amino)ethoxy)-N-(3,5-dichlorophenyl)isoxazole-5-carboxamide hydrochloride
[0134] 3-trifluoromethylaniline is replaced by 3,5-dichloroaniline, and all the other required raw materials, reagents and preparation methods are the same as in Example (8), to obtain the final product 3-(2-(cyclobutyl (methyl )amino)ethoxy)-N-(3,5-dichlorophenyl)isoxazole-5-carboxamide hydrochloride. 1 H NMR (400MHz, CDCl 3 )δ8.49(s,1H),7.61(s,2H),7.14(s,1H),6.64(s,1H),4.32(t,J=5.40 Hz,3H),2.91–2.77(m,1H ),2.66(t,J=5.40Hz,3H),2.16(s,3H),2.07–1.96(m,3H),1.94–1.78(m,2H),1.73–1.54(m,2H). 13 C NMR (101MHz, CDCl 3 )δ172.1, 162.6, 153.7, 138.3, 135.4, 125.3, 118.6, 99.9, 68.3, 60.5, 52.4, 38.6, 27.7, 13.8. HRMS (ESI): calcd for C 17 h 20 Cl 2 N 3 o 3 [M+H] + , 384.0876; found, 384.0905.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com