Preparation method of astragaloside acid
A technology of astragaloside IV and astragaloside IV is applied in the directions of steroids, organic chemistry, etc., and can solve the problems such as difficulty in obtaining pure astragaloside IV, complicated steps, side reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a brand-new preparation method of astragaloside. Specifically include the following steps:
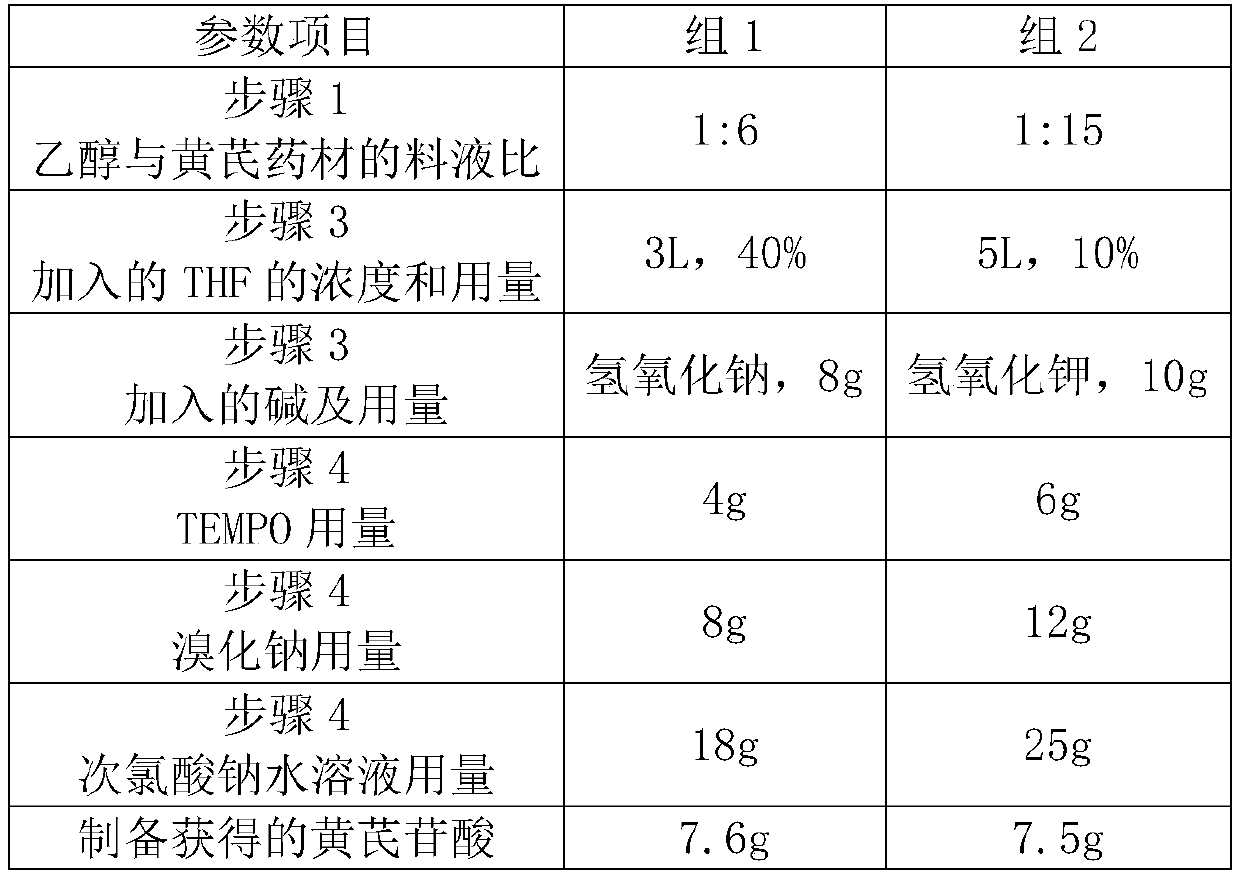
[0025] 1. Take the dry product of Astragalus membranaceus, crush it, and extract it three times with ethanol with a concentration of 30-95% at a solid-to-liquid ratio (g / mL) of 1:6-1:15, each time for 90 minutes.
[0026] 2. Combine the extracts, reclaim ethanol under reduced pressure, add water to dilute, and the solid-liquid ratio of water consumption to the quality of the Radix Astragali medicinal material is 0.5-2 (g / mL). After extracting twice with an equal volume of ethyl acetate, the extracted aqueous phase was passed through a D101 macroporous resin column. The quality of the resin column is the same as that of the Radix Astragali. Wash once with deionized water of 3 times the volume of the resin column, and then elute once with 70% ethanol of 3 times the volume of the resin column. The ethanol eluate was collected, and the ethanol was recovered...
Embodiment
[0032] Example: Preparation of Astragaloic Acid
[0033] 1. Take 10Kg of the dry product of Astragalus membranaceus, crush it, and extract it three times with 70% ethanol at a ratio of solid to liquid (g / mL) of 1:10, each time for 90 minutes.
[0034] 2. Combine the extracts, recover ethanol under reduced pressure, add water to dilute, the water consumption is based on the mass of Astragalus medicinal material, and the ratio of solid to liquid is 1:2 (g / mL). Pass the diluted aqueous solution through a D101 macroporous resin column, and the weight of the resin column is 10kg. Wash once with deionized water of 3 times the volume of the resin column, and then elute once with 70% ethanol of 3 times the volume of the resin column. The ethanol eluate was collected, and the ethanol was recovered under reduced pressure.
[0035] 3. Add 2 L of 50% tetrahydrofuran aqueous solution (THF) for dispersion. Subsequently, 10 g of sodium hydroxide was added, and alkaline hydrolysis was carr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com