Maturation of mucosal defense and gut/lung function in the preterm infant
A preterm, mucosal technique for the maturation of mucosal defense and gut/lung function in preterm infants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
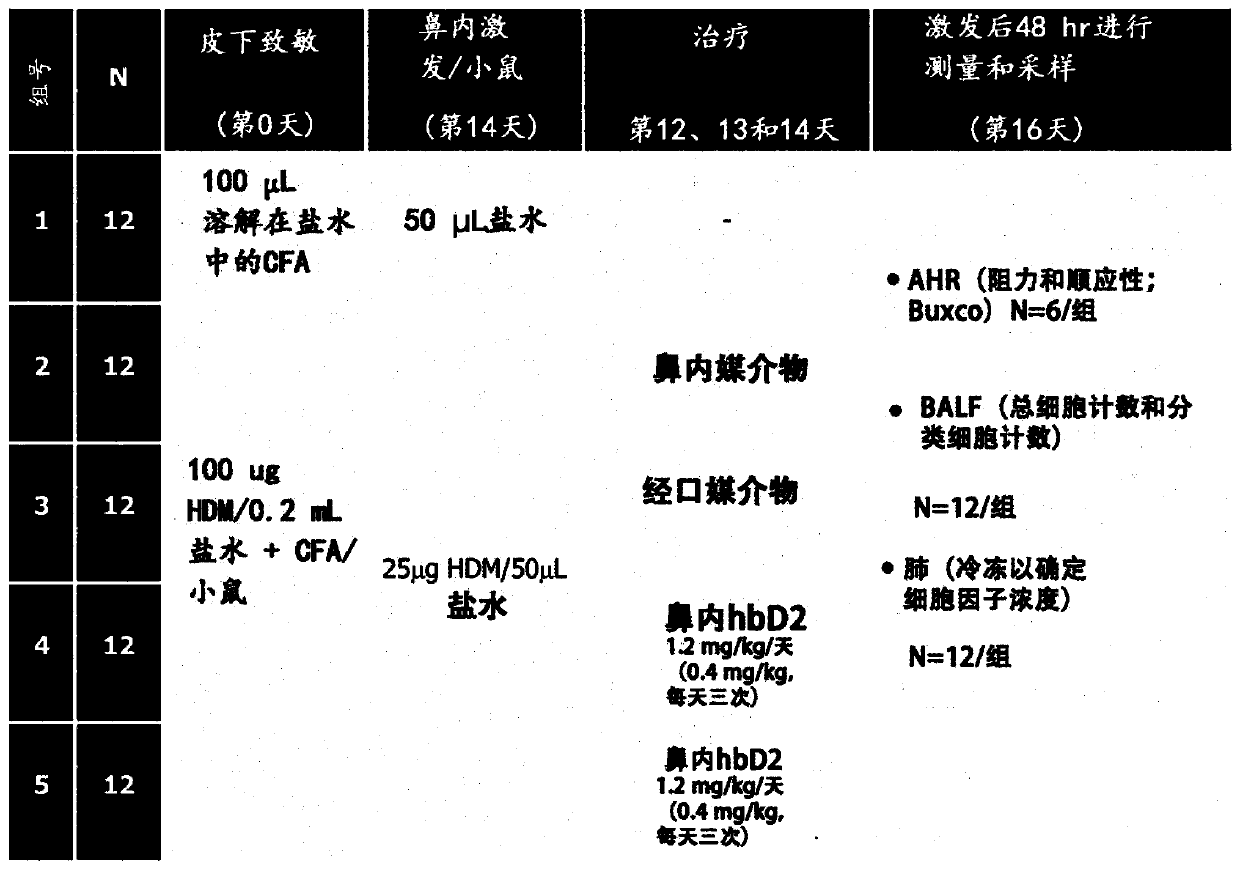
[0323] Example 1. Modulation of gut microbiota by prophylactic treatment with defensins.
[0324] Materials and methods:
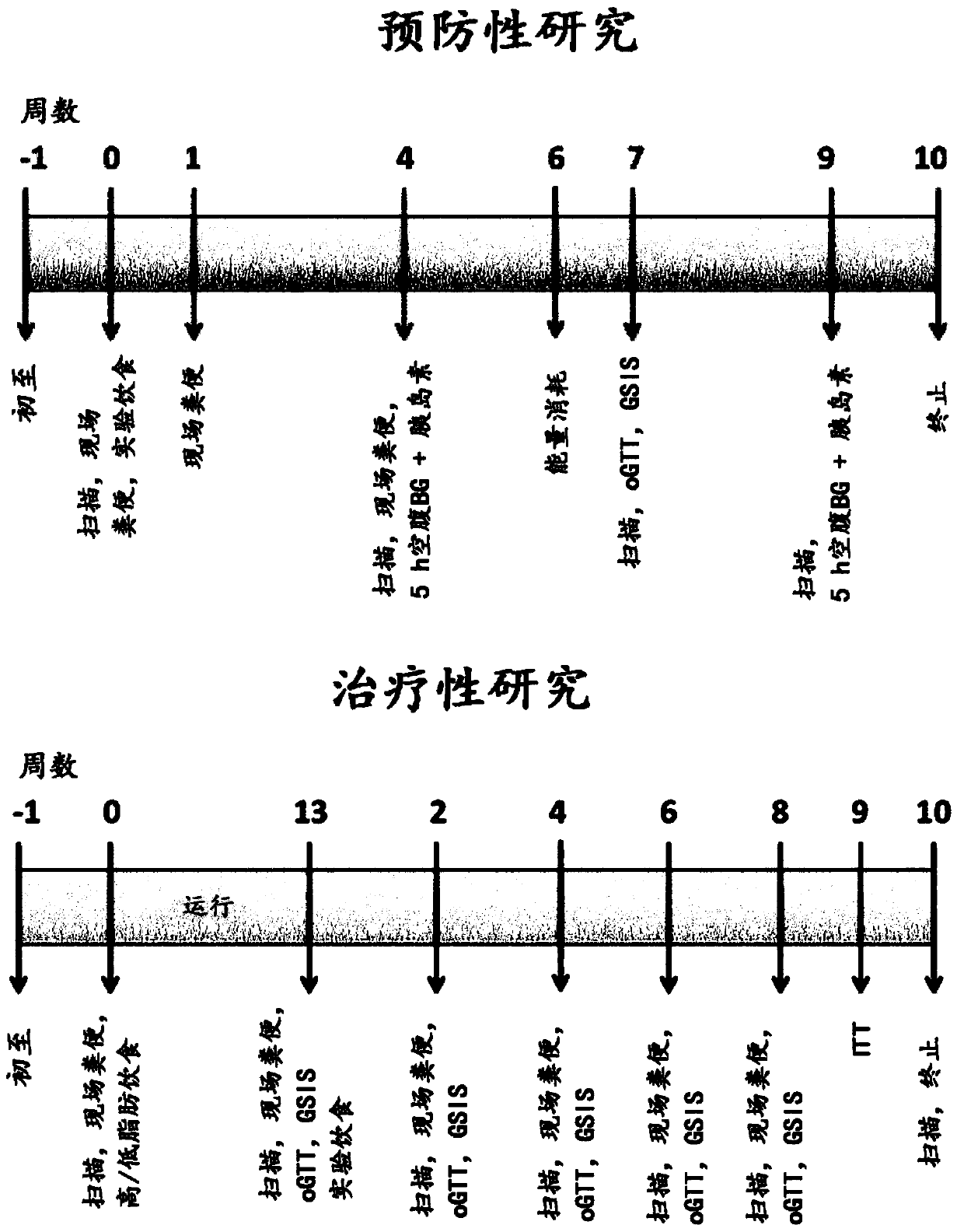
[0325] The overall experimental design is as figure 1 The above figure "Preventive Research" shows.
[0326] mouse: Mice were housed in triplicate, 4 cages per group. Feed intake was only registered daily before lights were turned off (at 6pm). Experimental procedures for grouping and cage order changes for individual mice. Mice were maintained at room temperature on a 12-h light / dark cycle under SPF standard conditions.
[0327] diet: For dosing, the average body weight was estimated to be 25 g per mouse. Each mouse eats approximately 3 grams of feed per day.
[0328] treatment solutions: Mice were fed a high-fat diet (HFD) or a low-fat (LF) control diet. HFD contained 4 subgroups; a) hBD-2, b) HD5, c) hBD-2 / HD5 and d) standard HFD without defensin supplementation. The defensin concentration was 1,2 mg hBD-2 per kg mouse per day. HD5 was a...
Embodiment 2
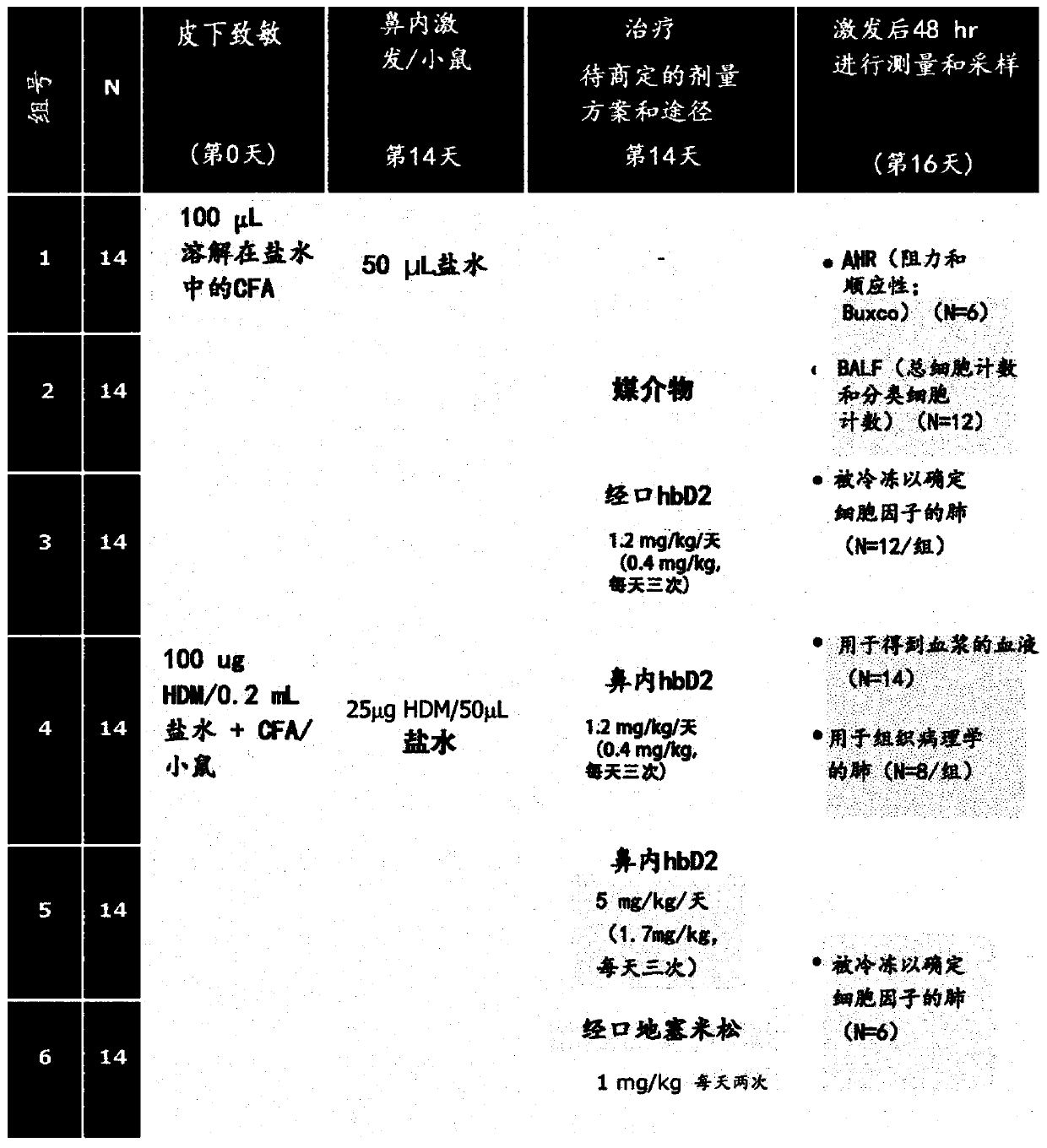
[0336] Example 2. Modulation of gut microbiota by intervention therapy with defensins.
[0337] Materials and methods:
[0338] The overall design of the study is as follows figure 1 The figure below "Treatment Studies" shows.
[0339] mice and diet . Experiments elucidate the role of hBD-2 and HD5 on the microbiota of diet-induced obese mice. The intervention was preceded by a 13-week run-in period in which mice were fed a very HFD (60% energy from fat). Only mice meeting the minimum 12 gram weight gain criterion (approximately 50% of initial body weight) during the run-in period were included in the final analysis. Mice that did not meet these criteria were kept in their respective cages as hierarchical "guardians". It was exposed to all experimental tests but excluded from analysis.
[0340] treatment solutions . Before intervention, MR scans were performed on all mice. Cages of mice were assigned to experimental groups based on their fat mass. All subsequent ...
Embodiment 3
[0350] Example 3. Method to determine the efficacy of prophylactic treatment with oral mammalian α-defensins and β-defensins in a preterm piglet model of necrotizing enterocolitis.
[0351] Materials and methods:
[0352] treatment solutions : 24 premature piglets were delivered by caesarean section at 105 days of gestation. Newborn piglets were immediately transferred to a temperature-regulated and oxygenated incubator. When breathing has stabilized, piglets are placed with umbilical and orogastric catheters. Parenteral nutrition was initially provided to all piglets via an esophageal tube. The enteral formula diet was made from three commercially available products for feeding infants 0-2 years of age.
[0353] Piglets were stratified according to birth weight and sex and divided into control and intervention groups receiving hBD-2.
[0354] test:
[0355] Signs of malaise or weakness (reluctance to stand, cold extremities, distended abdomen, dehydration, pallor, diar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com