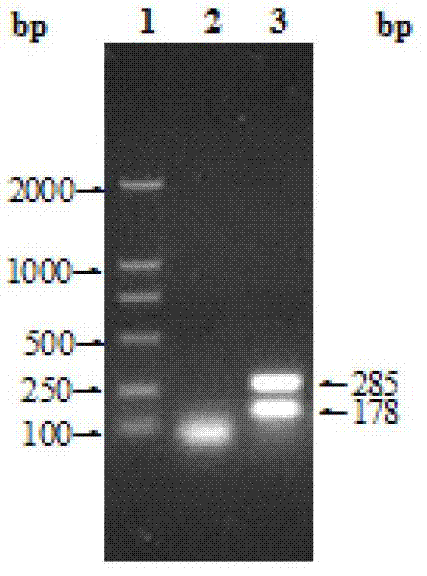
Animal brucellosis competition ELISA antibody test kit
A technology for brucellosis and brucellosis, applied in the field of biological product detection, can solve problems such as non-specificity, inability to effectively distinguish serum, loss, etc., to achieve the effect of improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] -Assembly of kit
[0059] Preparation of antigen-coated plates Use 0.05mol / L carbonate buffer (pH9.6, containing 0.01% thimerosal by volume) to dilute the LPS antigen to 1 μg / ml, and coat 100 / μl wells on the enzyme plate (Corning ), 2~8°C for more than 18h. The antigen coating solution in the well was discarded, and then blocked with 5% gelatin (containing 0.01% thimerosal) (dissolved in PBS, pH 7.4), 100 μL / well, 37°C for 2 hours.
[0060] After drying and discarding the blocking solution, dry at 37°C for 2 hours, add a desiccant and put it into a Xibo bag, and vacuum seal it.
[0061] Store at 2-8°C, and the validity period is tentatively set at 12 months.
[0062] The inspection packaging bag of the antigen-coated plate is well sealed, the bottom of the well is clean and transparent, and there is no foreign matter.
[0063] Dispensing of kit components
[0064] Strong positive control serum, weak positive control serum, negative control serum (provided by China V...
Embodiment 2
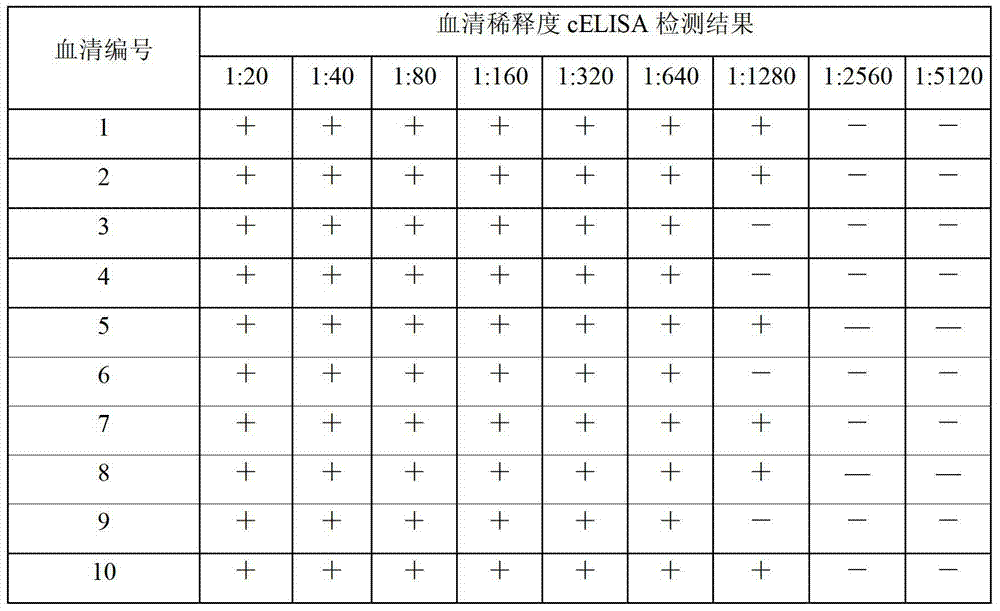
[0070] ———Kit sensitivity test
[0071]After the positive reference serum was serially diluted 1:2 to 1:32, 50 μL of each dilution was added to the antigen-coated wells, and then 50 μL of the monoclonal antibody diluted according to the requirements of the kit was added. The inhibition rate (PI) of the positive reference serum was ≥30% after 1:32 dilution. At the same time, strong positive serum control, weak positive serum control, negative serum control and serum blank control were set up. Two parallel wells were made for each sample. The serum PI of the strong positive control is 80%-110%; the PI of the weak positive control serum is 30%-70%; the PI of the negative control serum is 10%-15%. 450nm between 0.75 and 2.0.
[0072] After 10 parts of Brucella positive reference serum doubling dilutions, the Brucella antibody cELISA detection kit that laboratory trial-manufacture is carried out sensitivity test respectively, the cELISA kit prepared by the present invention dete...
Embodiment 3
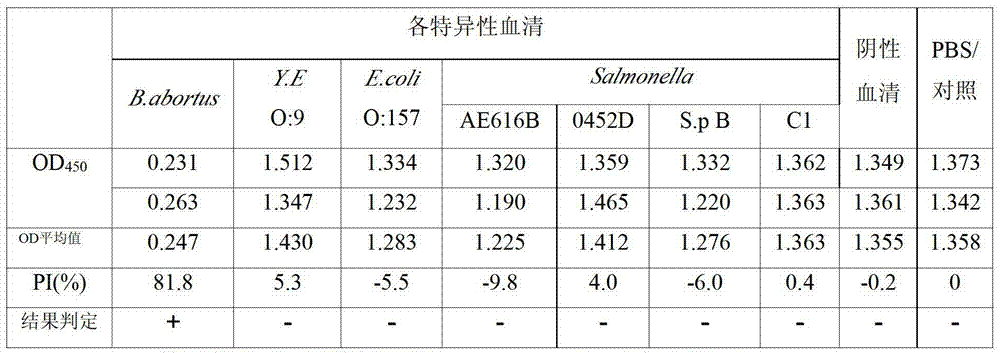
[0076] ———— Kit specificity test
[0077] Yersinia enterocolitica O:9 (Y.E.O:9), Salmonella (S.E.) 045-2 (Group D), C500 (Group C1), AE616 (Group B), Paratyphi B (S.paratyphiB ) and Escherichia coli O:157 (933 strains) positive sera, as well as 30 negative reference sera, were used as test samples for cELISA to verify its specificity. Among them, Yersinia enterocolitica O:9 (Y.E.O:9), Salmonella (S.E.) 045-2 (Group D), C500 (Group C1), AE616 (Group B), and Bacillus paratyphi (S.paratyphi B) and 30 copies of negative reference serum were provided by the Bacterial Products Testing Office of China Veterinary Drug Control Institute, and Escherichia coli O:157 (933 strains) positive serum was provided by Professor Gao Song of the Veterinary College of Yangzhou University.
[0078] Results The kit prepared by the present invention detects the positive serum of Yersinia enterocolitica O: 9, and the positive serum of Escherichia coli O: 157, and its PI is less than 6%; detects Salmon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com