Synthesis method of light controlled-release compound and application of light controlled-release compound in tumor treatment
A synthesis method and compound technology, applied in the field of photo-controlled release compound synthesis, can solve the problems of tumor insensitivity, poor diagnosis and treatment effect, single imaging and treatment mode, etc., and achieve the effect of improving curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The content of the present invention will be described in detail below in conjunction with specific embodiments. It should be noted that what follows is an explanation rather than a limitation of the present invention.
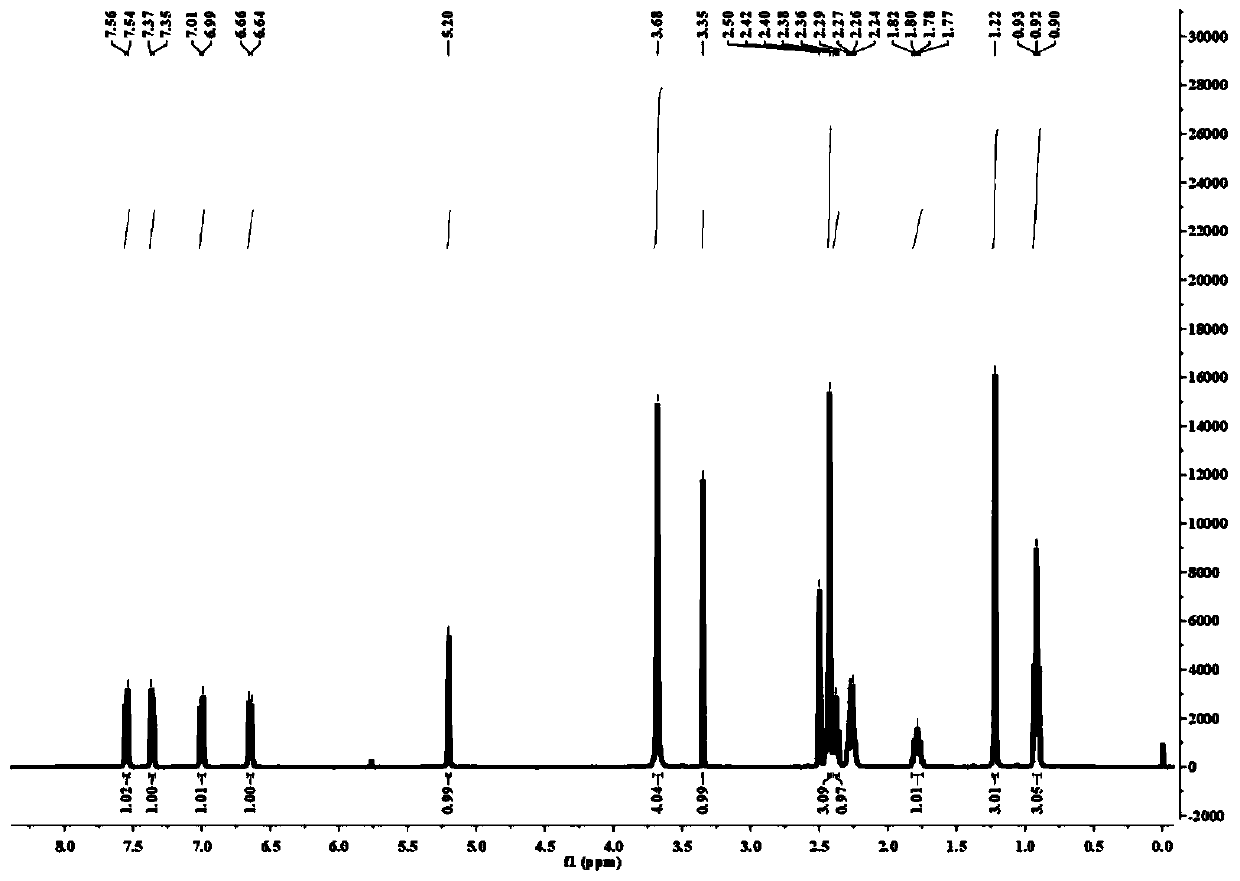
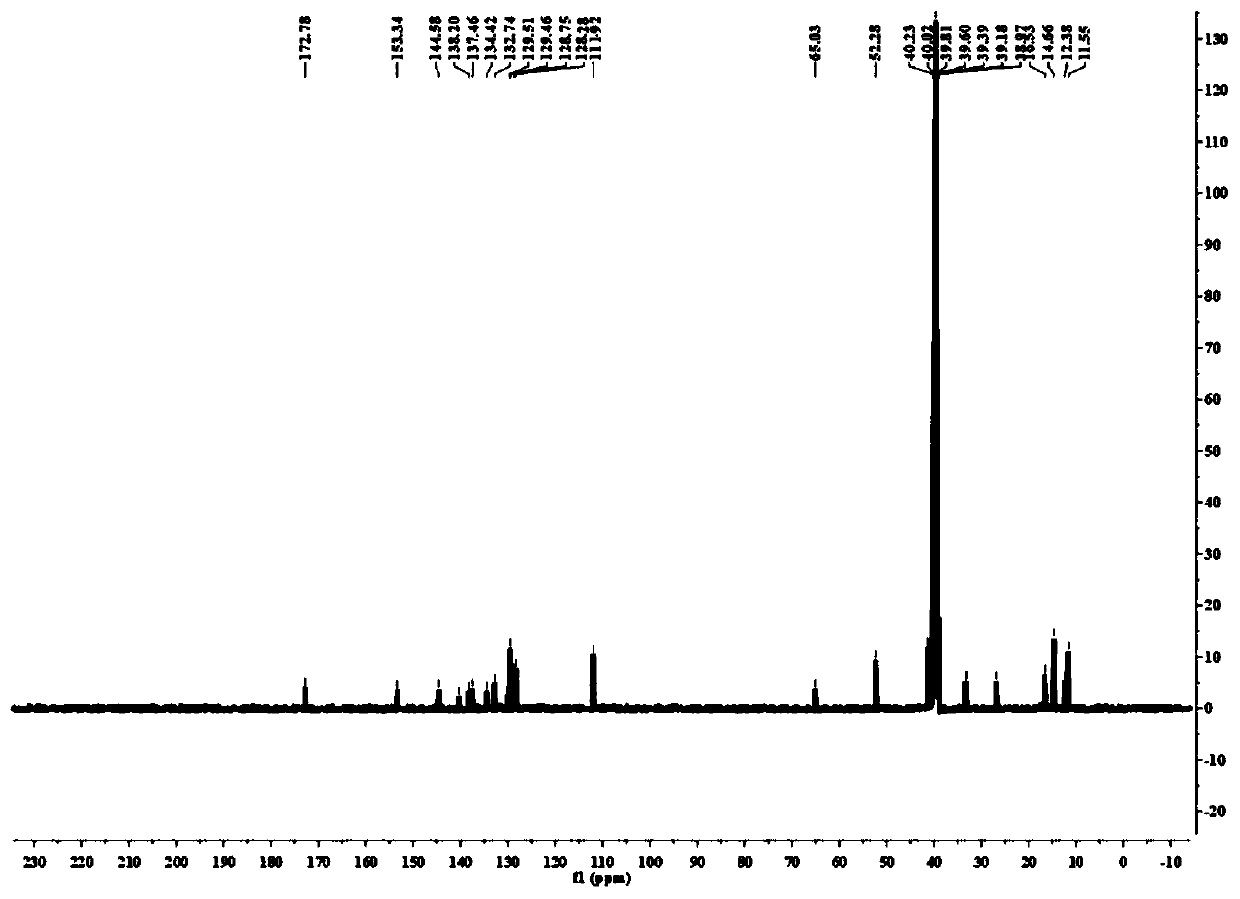
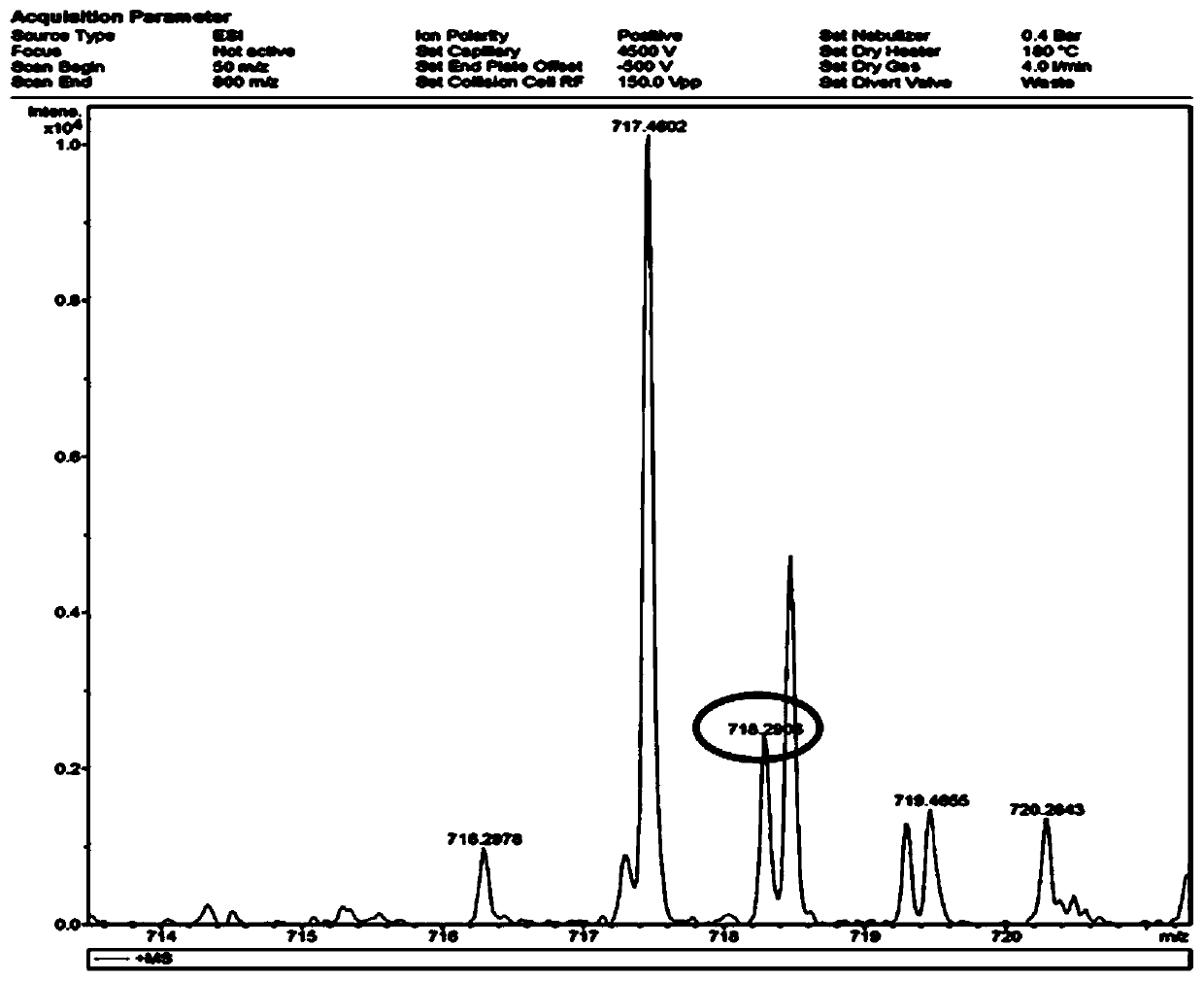
[0033] 1. The synthetic method of the light-controlled release compound of the present invention;
[0034]Including the following steps: (i) 4-benzoyl chloride (2mmol) was dissolved in a round-bottomed flask containing 100mL of dichloromethane, and argon was introduced into the round-bottomed flask for 15min, and 2,4-dimethyl- 4mmol of 3-ethyl-1H-pyrrole, then a small amount of trifluoroacetic acid was added dropwise, the mixture was stirred at room temperature for about 12h, 4mmol of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone was added, After continuing to stir for 4h, add 10mmol of triethylamine and continue to stir for 15min. Slowly add 15mmol of boron trifluoride ether in an ice bath. After the dropwise completion, the mixture is stirred overnight a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com