Generic inert carrier escherichia coli and potential application thereof
An inert carrier, Escherichia coli technology, applied in the field of biomedical technology detection, can solve problems such as non-agglutination, and achieve the effects of perfecting poor specificity, improving poor specificity, huge application value and market prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Obtaining and verifying the ubiquitous inert vector Escherichia coli SE1H
[0040] The inert carrier Escherichia coli SE1 (preservation number CGMCC No.17339) was inoculated into LB liquid medium and shaken at 37°C for 12 hours. Then 30μL of bacterial liquid was drawn and streaked in LB solid medium, and cultured at 37°C for 16-18 hours. Obtain the second generation of colonies, pick a single colony of the second generation and inoculate it in LB liquid medium, use LB liquid and solid medium as a cycle to alternate culture. Starting from the 40th generation, the single colony obtained is a ubiquitous inert carrier bacteria SE1H, in fact, by continuing to pass down from the 41st to the 60th generation, any of them will also have the characteristics of the ubiquitous inert carrier bacteria SE1H.
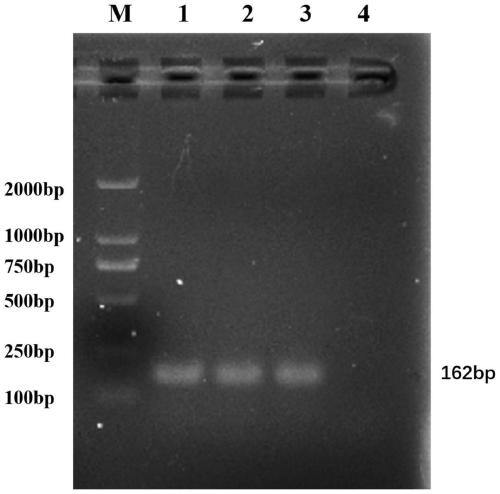
[0041] Take 1 mL of the above-mentioned overnight cultured SE1H bacterial liquid and prepare DNA template by boiling method. The E. coli β-glucuronidase gene uidA was am...
Embodiment 2
[0049] Example 2 Test for non-specific agglutination of pan-type inert carrier Escherichia coli SE1H with human and animal serum and whole blood of different backgrounds
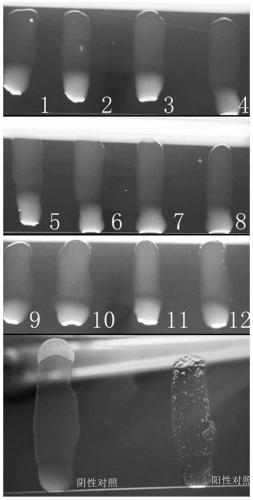
[0050] The Escherichia coli SE1H bacterial solution obtained by overnight culture in Example 1 was centrifuged at 4°C at 4000 rpm for 10 minutes, the supernatant was discarded, and the bacterial sludge was resuspended in sterile normal saline, centrifuged and washed three times, and then resuspended to a concentration gradient of different concentrations of bacteria. Before the test, mix the bacterial liquid with a vortexer, and perform agglutination test with sterile normal saline and SPF chicken serum to ensure that the test bacterial liquid does not self-agglomerate or non-specific agglutination. Take several common glass plates with clean surfaces in an ultra-clean bench (20℃~25℃), centrifuge and resuspend the carrier bacteria with sterile pre-cooled PBS to 4℃ for 3 times, then resuspend and dilute to the sp...
Embodiment 3
[0056] Example 3 Test and verification of the surface expression of the pan-type inert carrier bacteria Escherichia coli SE1H bacteria and the ability to carry the antigenic factor P of avian Salmonella
[0057] (1) Amplification of the coding gene p of Avian Salmonella expressing antigen factor P
[0058] According to the complete genome sequence of Salmonella pullorum ATCC 9120 strain in NCBI GenBank (NCBI accession number: CP012347.1), the complete genome sequence of Salmonella pullorum S44987_1 strain (NCBI accession number: LK931482.1), and the complete genome sequence of Salmonella pullorum S06004 strain ( NCBI accession number: CP006575.1), Salmonella pullorum QJ-2D-Sal strain complete genome sequence (NCBI accession number: CP022963.1), Salmonella typhimurium 287 / 91 strain complete genome sequence (NCBI accession number: AM933173.1) , Salmonella typhimurium 9184 strain (NCBI accession number: CP019035.1) published the full length of the genome sequence, respectively, search...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com