Application of chlorogenic acid, isomer or pharmaceutically acceptable salt in preparation of tumor chemotherapy sensitization drugs
A technology of isomers and chemotherapeutic drugs, which is applied in the application field of preparing tumor chemotherapeutic sensitizing drugs, can solve the problems of low solubility of chlorogenic acid, limit the clinical application of chlorogenic acid, and limit the value of clinical application, so as to reduce the Incidence of toxic and side effects, increased safety and therapeutic effect, and improved therapeutic sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
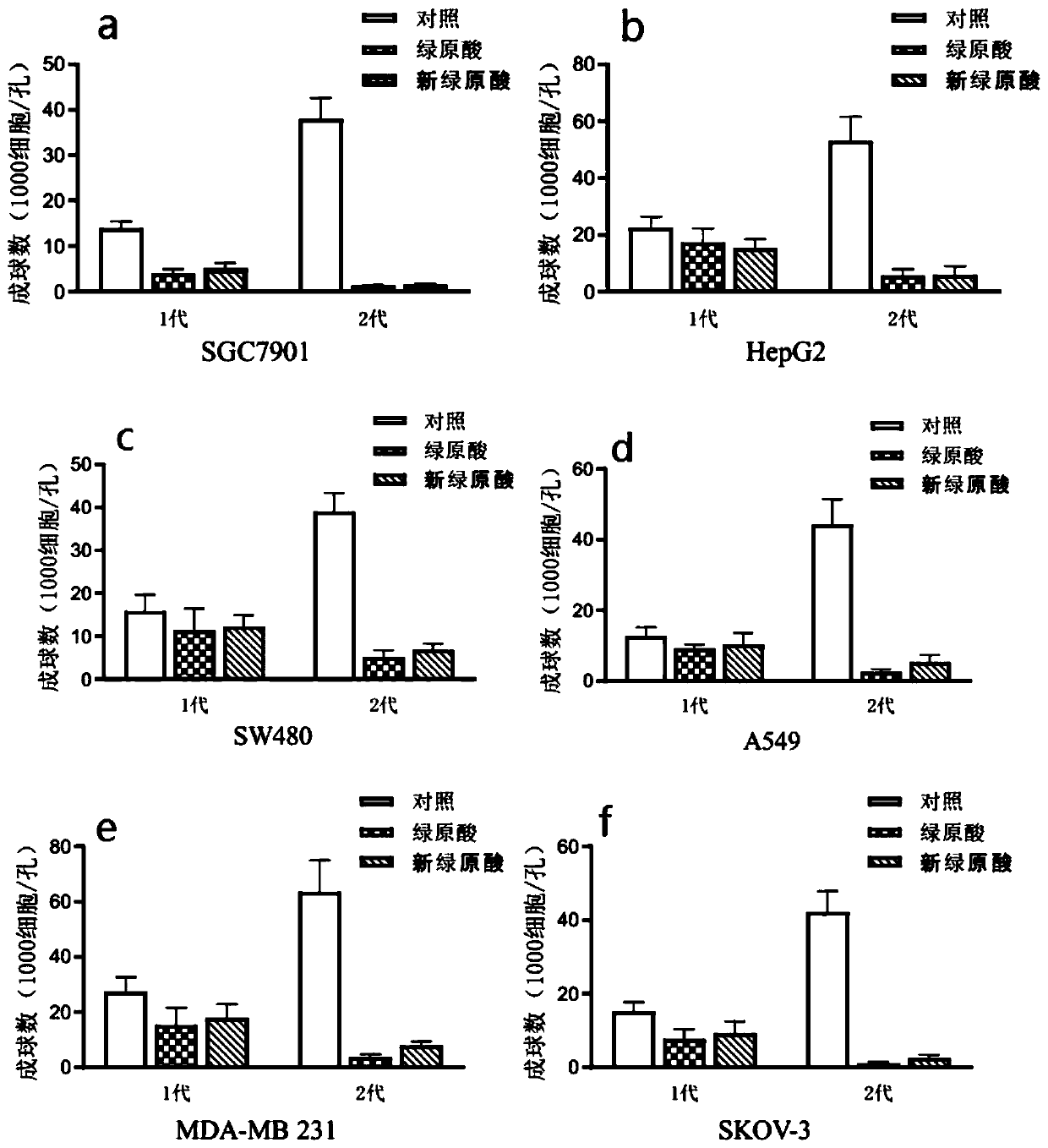
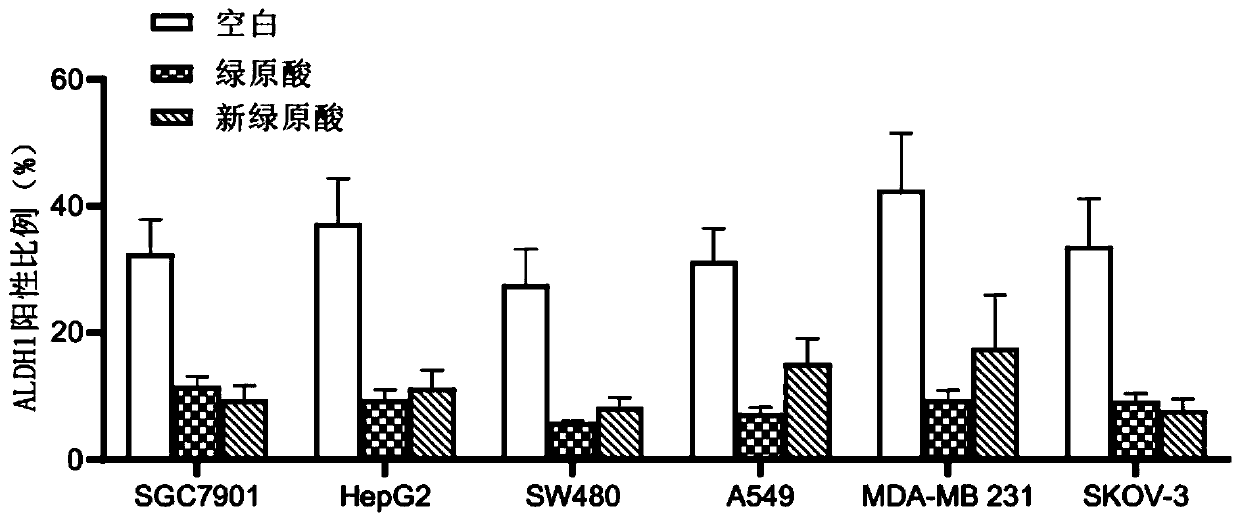
Examples
Embodiment
[0041] 1. Obtaining chlorogenic acid and its isomers
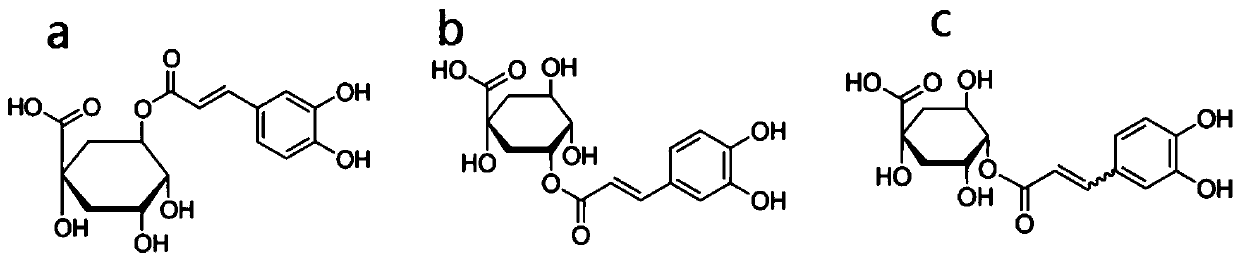
[0042] Chlorogenic acid and its isomers can be purchased from commercial products or synthesized with reference to previous patents. The compound structures of chlorogenic acid and neochlorogenic acid are as follows: figure 1 Shown (where a is chlorogenic acid, b is neochlorogenic acid, and c is cryptochlorogenic acid). At the same time, in order to enhance the stability and solubility of chlorogenic acid and its isomers, the preparation process (including: pharmaceutical carrier, absorption enhancer) can be used to improve drug stability, improve oral absorption rate, increase target tropism; pharmaceutical carriers include but not limited to microcapsules, microspheres, nanoparticles, liposomes, etc., absorption enhancers include but not limited to cholic acid and its salts (such as sodium deoxycholate), surface active Agents (such as poloxamer), polymer materials (such as carbomer), etc.), improve the bioavailability ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com