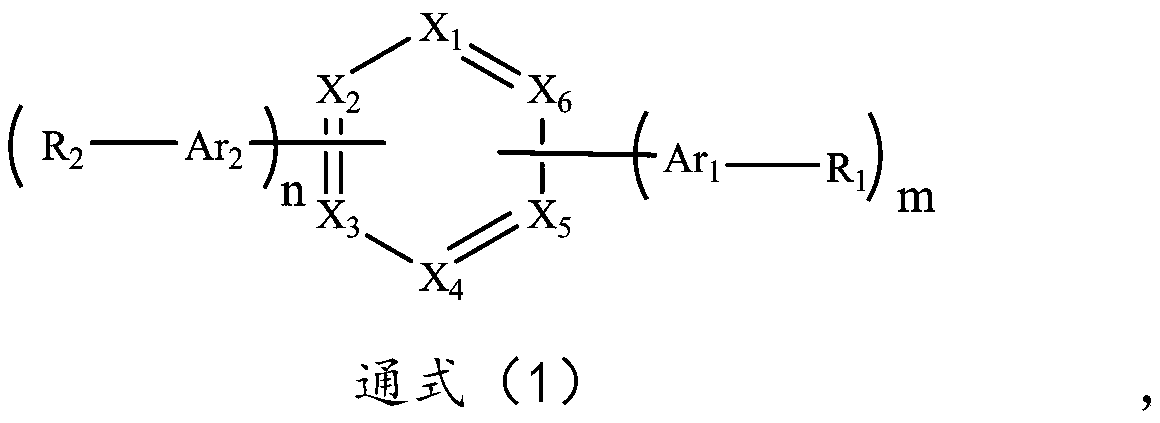
Organic compound based on azabenzene and benzoheterocycle, and preparation method and application of organic compound
A technology of organic compounds and benzoheterocycles, which is applied in the fields of organic chemistry, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems affecting the angular distribution of OLED radiation spectrum and complex manufacturing process, etc., so as to improve the light extraction efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
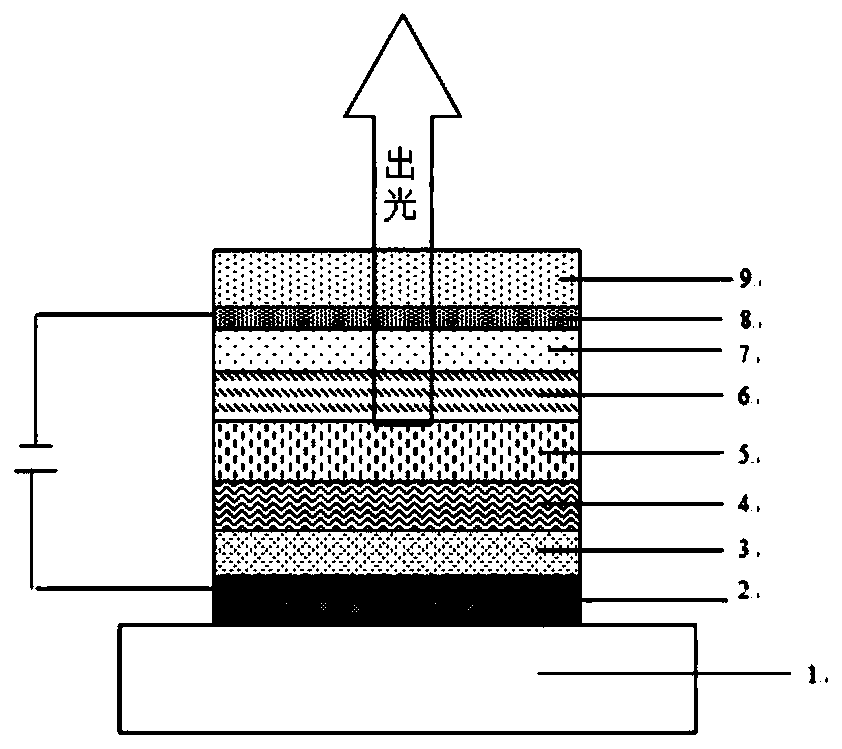
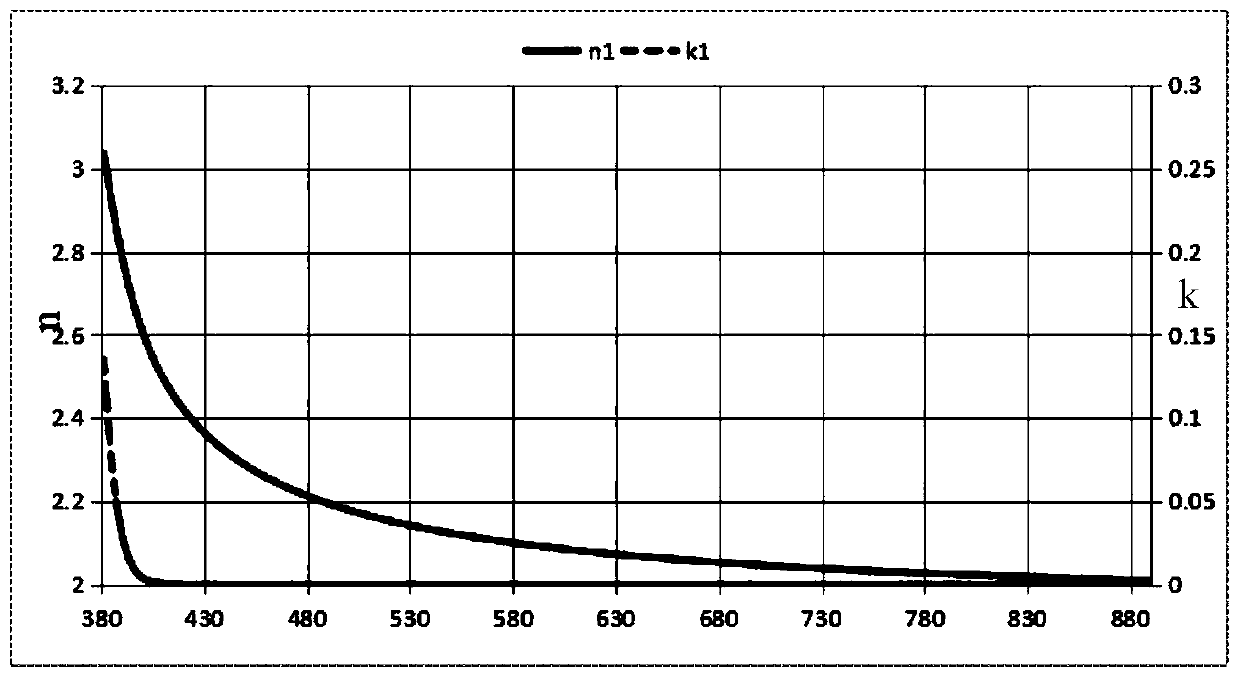
Image
Examples
Embodiment 1
[0067] Embodiment 1: the synthesis of compound 1:
[0068]
[0069] In a 250mL three-necked flask, nitrogen gas was introduced, 0.02mol of raw material A-1, 150ml of DMF, 0.024mol of raw material B-1 and 0.0002mol of palladium acetate were added, stirred, and then 0.03mol of K 3 PO 4 The aqueous solution was heated to 130°C, refluxed for 10 hours, sampled and plated, and the reaction was complete. Natural cooling, adding water, filtering the mixture and drying in a vacuum oven, the resulting residue was purified by silica gel column to obtain compound intermediate M-1;
[0070] In a 250mL three-necked flask, nitrogen gas was introduced, 0.01mol of intermediate M-1, 150ml of DMF, 0.03mol of raw material C-1 and 0.0002mol of palladium acetate were added, stirred, and then 0.03mol of K 3 PO 4 Aqueous solution, heated to 130°C, refluxed for 24 hours, sampled and plated, the reaction was complete. Cool naturally, add water, filter the mixture and dry in a vacuum oven, and th...
Embodiment 2
[0071] Embodiment 2: the synthesis of compound 5:
[0072]
[0073] Prepared by the synthetic method of compound 1 in embodiment 1, difference is to replace raw material A-1 with raw material A-2, raw material C-2 replaces raw material C-1; Elemental analysis structure (molecular formula C 50 h 35 N 3 o 2 S 2 ): Theoretical value: C, 77.59; H, 4.56; N, 5.43; O, 4.13; S, 8.28; HPLC-MS: The molecular weight of the material is 773.22, and the measured molecular weight is 773.95.
Embodiment 3
[0074] Embodiment 3: the synthesis of compound 9:
[0075]
[0076] Prepare by the synthetic method of compound 1 in embodiment 1, difference is to replace raw material C-1 with raw material C-3; Elemental analysis structure (molecular formula C 53 h 40 N 2 S 4 ): theoretical value: C, 76.41; H, 4.84; N, 3.36; S, 15.39; test value C, 76.41; H, 4.85; N, 3.36; HPLC-MS: The molecular weight of the material is 832.21, and the measured molecular weight is 832.22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com