Method for selectively recycling rhenium from high-arsenic waste acid system
A selective and systematic technology, applied in the direction of improving process efficiency, can solve the problems of poor selective extraction performance and low separation rate of rhenium and arsenic, and achieve the effect of low cost, simple process flow and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
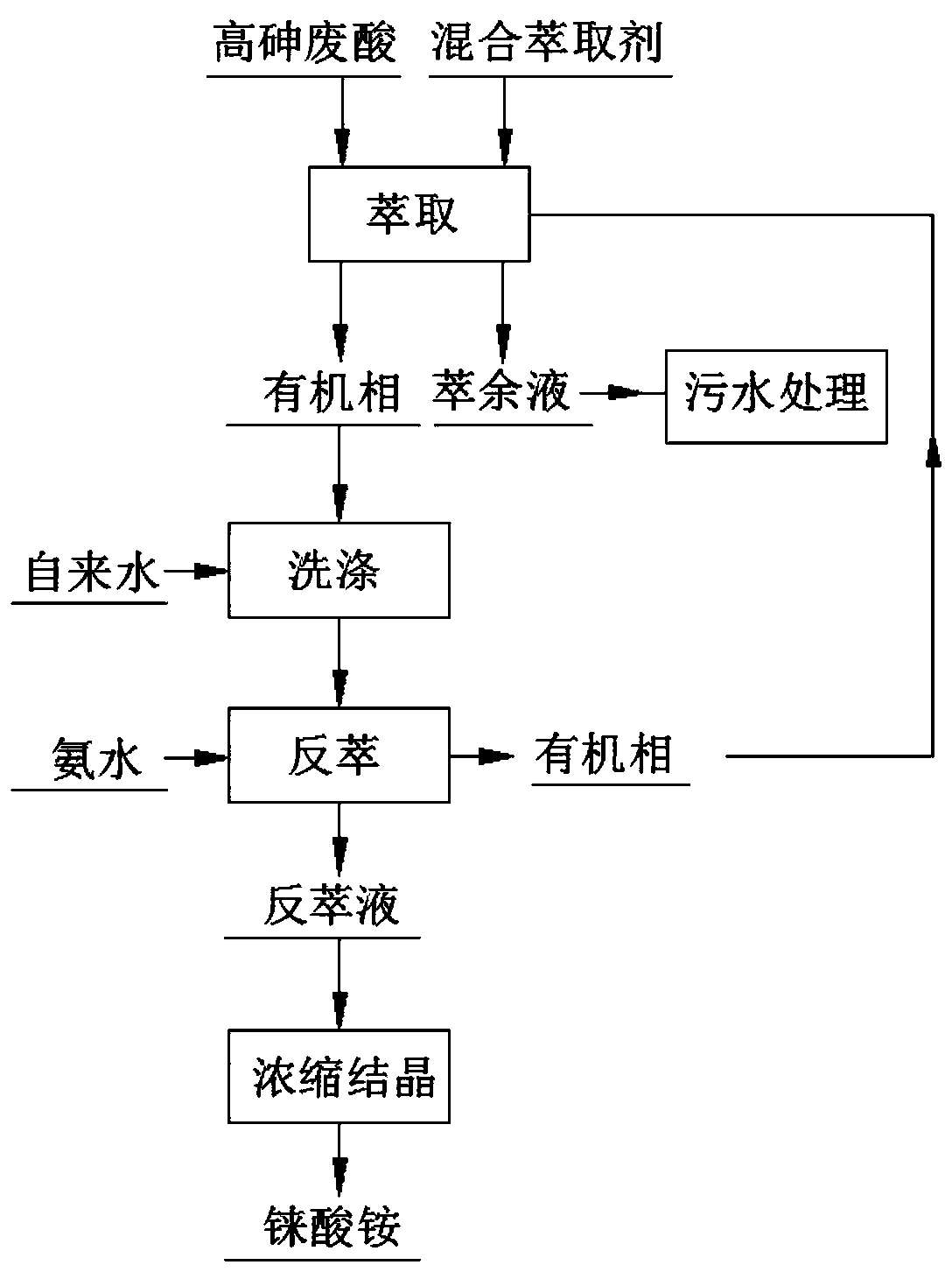
[0026] A method for selectively recovering rhenium from a high-arsenic waste acid system containing H + 0.38mol / L, As6.62g / L, Fe 39.08mg / L, Pb 17.22mg / L, Zn 271.75mg / L, Re 42.5ug / L, Cl - 1.97g / L, F - 0.1g / L,SO 4 2- 13.64g / L, including the following steps:
[0027] Use a mixed extractant to directly extract high-arsenic waste acid to obtain a rhenium-rich organic phase; the mixed extractant includes trioctylamine, N1923, isoamyl alcohol and sulfonated kerosene, the quality of trioctylamine, N1923, isoamyl alcohol and sulfonated kerosene The percentages are 10%, 4%, 18% and 68% respectively; during extraction, O / A=1:2, extraction time 10min, single-stage extraction, room temperature. It is determined that in this step, the extraction rate of rhenium is 99.2%, and the separation rate of rhenium and arsenic is 99.46%.
[0028] In this embodiment, the separation rate of rhenium and arsenic is (1-(the amount of arsenic in the collected rhenium material / the amount of arsenic in ...
Embodiment 2
[0030] Compared with Example 1, this example differs in that sodium hyposulfite is added to the high-arsenic waste acid before using the mixed extractant to extract the high-arsenic waste acid, and the amount of sodium hyposulfite added is 1.1 times the theoretical amount for reducing arsenic.
[0031] It is determined that in this step, the extraction rate of rhenium is 99.2%, and the separation rate of rhenium and arsenic is 99.78%.
Embodiment 3
[0033] A method for selectively recovering rhenium from a high-arsenic waste acid system containing H + 0.52mol / L, As6.25g / L, Fe 41.2mg / L, Pb 17.32mg / L, Zn 255.6mg / L, Re 287.1ug / L, Cl - 2.7g / L, F - 0.13g / L,SO 4 2- 16.64g / L, including the following steps:
[0034] Use a mixed extractant to directly extract high-arsenic waste acid to obtain a rhenium-rich organic phase; the mixed extractant includes trioctylamine, N1923, isoamyl alcohol and sulfonated kerosene, the quality of trioctylamine, N1923, isoamyl alcohol and sulfonated kerosene The percentages are 14%, 5%, 12% and 69% respectively; during extraction, O / A=1:2, extraction time 10min, three-stage extraction, normal temperature. It is determined that in this step, the extraction rate of rhenium is 99.61%, and the separation rate of rhenium and arsenic iron is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com