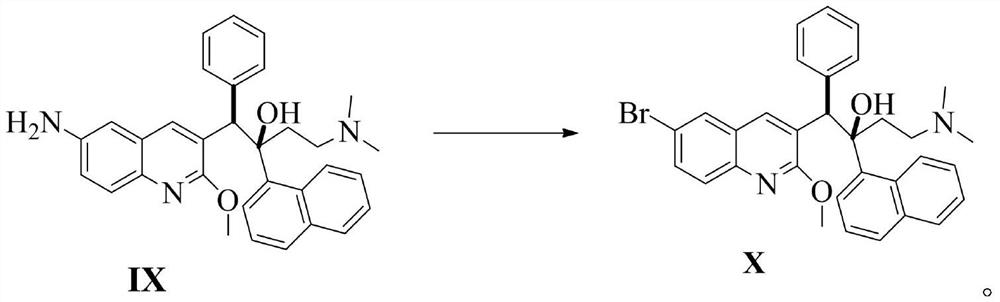
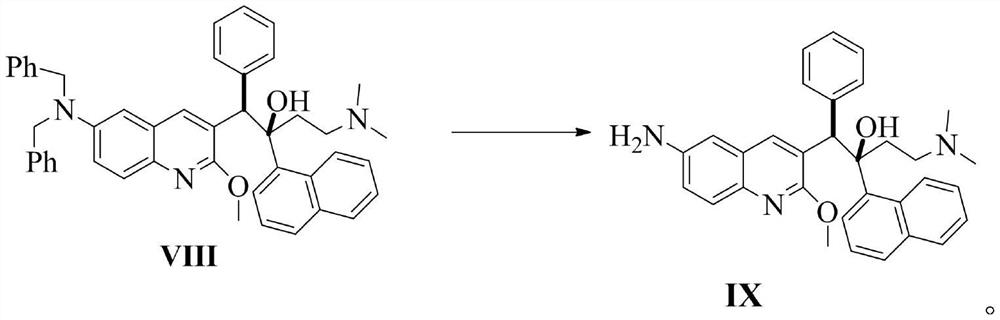
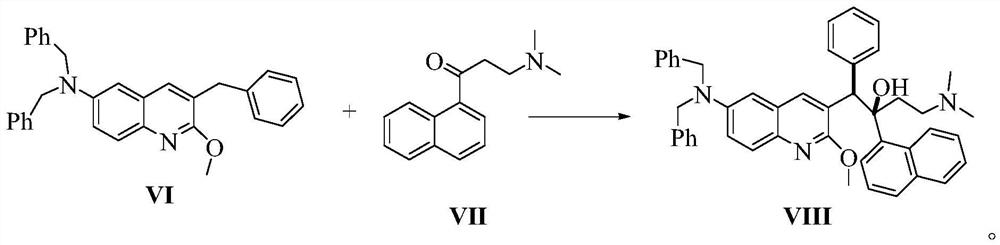
Preparation method of bedaquiline and intermediate thereof
A technology of quinoline racemate and betadine, which is applied in the field of pharmaceutical synthesis, can solve the problems of low reaction temperature, high energy consumption, expensive reaction conditions of reagents, etc., and achieves a technology that reduces production cost, improves reaction yield, and is easy to crystallize and purify. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Embodiment 1: the preparation of N-(4-aminophenyl) phthalimide (compound I)
[0125]
[0126] Referring to the method recorded in the document CN103834051A (see the synthesis of amines in page 3 (2) of the description), it is prepared by the following method:
[0127] Dissolve phthalic anhydride (14.g) in DMF, add p-phenylenediamine (14g, 1.3eq) in batches, raise the temperature to 150°C for reaction, maintain the temperature for 3 hours, and cool down after the reaction is complete. The reaction mixture was slowly poured into ice water, and a solid precipitated out, which was filtered by suction to obtain compound I (17.1 g, yield 72%). 1 H NMR (400MHz, DMSO-d 6 )δ: 7.86-7.93 (m, 4H), 7.02 (d, J = 8.0Hz, 2H), 6.64 (d, J = 8.0Hz, 2H), 5.33 (s, br, 2H); Ms (+C, ESI): M=238, measured value: (239, M+1).
Embodiment 2
[0128] Example 2: Preparation of N-(4-(1,3-diketoisoindol-2-yl)phenyl)-3-phenylpropanamide (compound II)
[0129]
[0130] Compound I (23.8g, 1.1eq) was dissolved in 70ml of toluene, triethylamine (20.9ml, 1.5eq) was added, and the reaction mixture was cooled in ice water. Phenylpropanoyl chloride (17.6g, 1.05eq) was slowly added dropwise to the toluene solution of compound I, and after the addition was completed, the temperature was raised naturally, and stirred overnight at room temperature. After the reaction was completed, 200ml of water was added to the reaction mixture at room temperature, stirred for 2h, filtered, and the filter cake was washed with 20ml*3 water and 20ml of toluene, and dried to obtain compound II (33g, yield 89%).
[0131] 1 H NMR (400MHz, DMSO-d 6 )δ10.09(s,1H),7.87-7.97(m,5H),7.71(d,J=8.0Hz,2H),7.36(d,J=8.0Hz,2H),7.19-7.32(m,2H ), 7.03(d, J=8.0Hz, 1H), 6.67(d, J=8.0Hz, 1H), 2.94(t, J=8.0Hz, 2H), 2.67(t, J=8.0Hz, 2H); Ms(+C, ESI): M=370, measur...
Embodiment 3
[0132] Example 3: Preparation of 2-(3-benzyl-2-chloro-quinolin-6-yl)isoindole-1,3-dione (compound III)
[0133]
[0134] DMF (29ml, 5eq) was placed in a round bottom flask, POCl was added dropwise under ice water cooling 3 (50ml, 7eq), after the dropwise addition, continue to stir in ice water for 10min. The ice-water bath was removed, and stirring was continued at room temperature for 10 min. Add 80ml of acetonitrile and solid compound II (37g, 1eq), and heat to 80°C for 8h. After the reaction was completed, the reaction solution was poured into a sodium carbonate solution mixed with ice and water, stirred for 30 min, filtered with suction, washed with water, crystallized with ethanol, and dried to obtain compound III (27.1 g, yield 68%).
[0135] 1 H NMR (400MHz, DMSO-d 6 )δ8.43(s,1H),8.10-8.11(m,2H),8.02-8.03(m,2H),7.94-7.96(m,2H),7.89-7.90(m,1H),7.25-7.34( m, 5H), 4.27 (s, 2H); Ms (+C, ESI): M=398, found value: (399, M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com