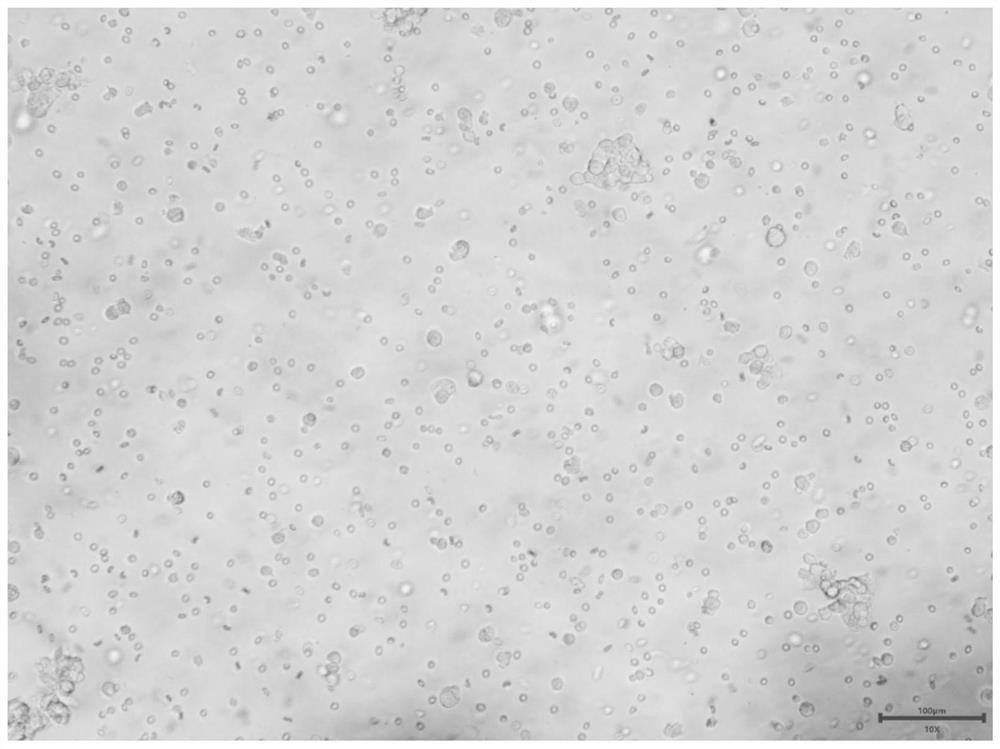
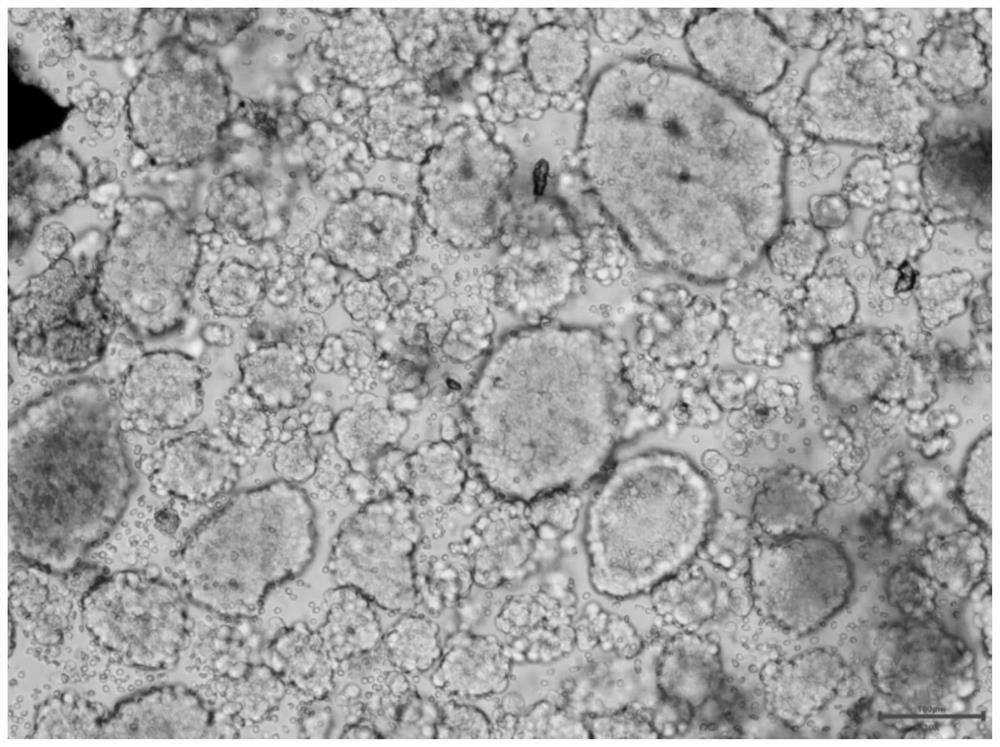
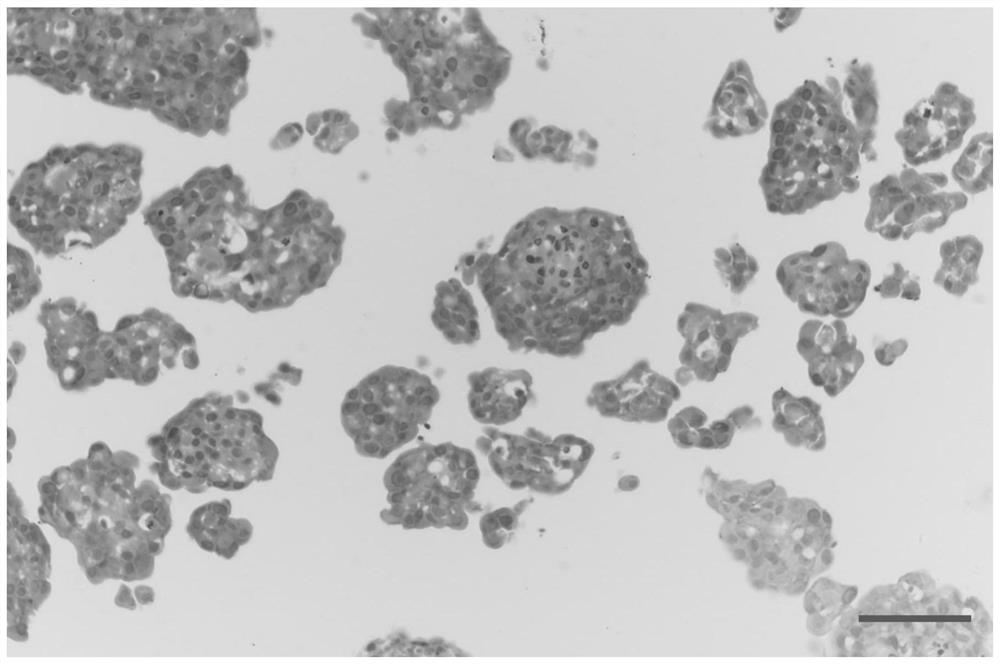
Culture method of gynecological tumor primary cells
A technology for gynecological tumors and primary cells, which is applied in the field of culture of primary cells for gynecological tumors, and can solve the problems of long culture period, difficult removal of miscellaneous cells, and low success rate of culture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1. Preparation of reagents for culturing primary cells of gynecological tumors
[0083] 1. Sample preservation solution (100mL)
[0084] The specific formula of the sample preservation solution (100mL) is shown in Table 1.
[0085] Table 1 Sample Preservation Solution (100mL)
[0086]
[0087]
[0088] After the sample preservation solution is prepared, it is divided into 15mL centrifuge tubes, 5mL per tube. After aliquoting, it can be stored at 4°C for 1 month.
[0089] 2. Sample cleaning solution (100mL)
[0090] The specific formula of the sample cleaning solution (100mL) is shown in Table 2.
[0091] Table 2 Sample cleaning solution (100mL)
[0092]
[0093] The sample cleaning solution should be prepared and used immediately.
[0094] 3. Sample dissociation solution (10mL)
[0095] The specific formulation of the sample dissociation solution (10mL) is shown in Table 3.
[0096] Table 3 Sample Dissociation Solution (10mL)
[0097]
[0098]...
Embodiment 2
[0177] Example 2. Obtaining of postoperative specimens / biopsy puncture specimens / pleural and ascites samples of gynecological tumors
[0178] 1. Cooperate with tertiary hospitals, and the cooperation has passed the formal medical ethics review.
[0179] 2. The attending doctor selects the patients according to the clinical indications stipulated in the medical guidelines, and selects appropriate samples for in vitro culture according to the clinical indications during the operation. The selection criteria of the samples are: primary breast cancer, ovarian cancer, uterine Endometrial cancer, cervical cancer or its metastatic lesions, surgical specimens weighing more than 20mg, or pleural and ascites samples exceeding 100mL, or needle biopsy specimens exceeding 4 samples.
[0180] 3. The attending doctor provides basic clinical information such as the patient's gender, age, medical history, family history, smoking history, pathological staging and typing, and clinical diagnosis....
Embodiment 3
[0182] Example 3, Gynecological Tumor Solid Tumor Tissue Sample Dissociation Pretreatment
[0183] The following operations need to be performed on ice, and the entire operation steps need to be completed within 10 minutes.
[0184] The surgical equipment used in the following operations must be sterilized by high temperature and high pressure before use.
[0185] 1. Sample weighing.
[0186] 2. Clean the surface of the sample with 75% (volume percent) ethanol for 10 to 30 seconds.
[0187] 3. Wash the sample 5 times with sample cleaning solution, and wash the sample 5 times with sterile PBS solution.
[0188] 4. Use ophthalmic scissors, ophthalmic forceps, scalpel and other equipment to carefully peel off the adipose tissue, connective tissue, and necrotic tissue in the sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com