Pantoprazole sodium freeze-drying preparation, preparation method thereof, and injection preparation prepared from pantoprazole sodium freeze-drying preparation
A technology for pantoprazole sodium and freeze-dried preparations, which is applied in the field of pharmaceutical preparations, can solve the problems of long freeze-drying time and instability of pantoprazole sodium freeze-dried preparations, and achieve stable product quality, clarity and color stability , Improving the effect of molding and color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
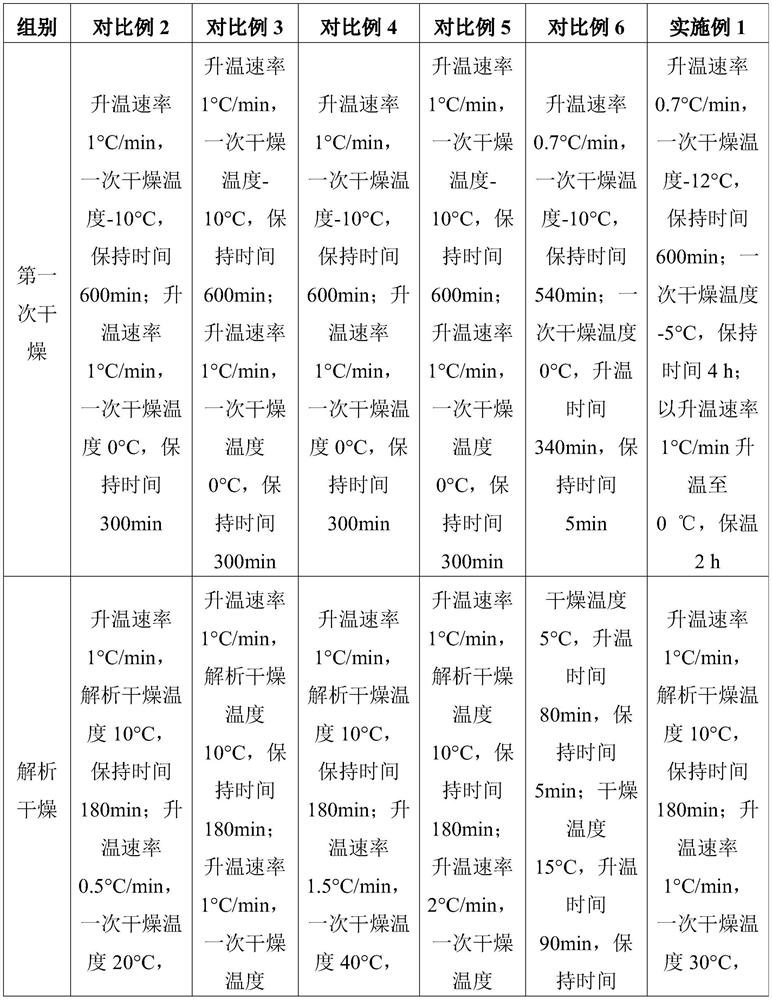
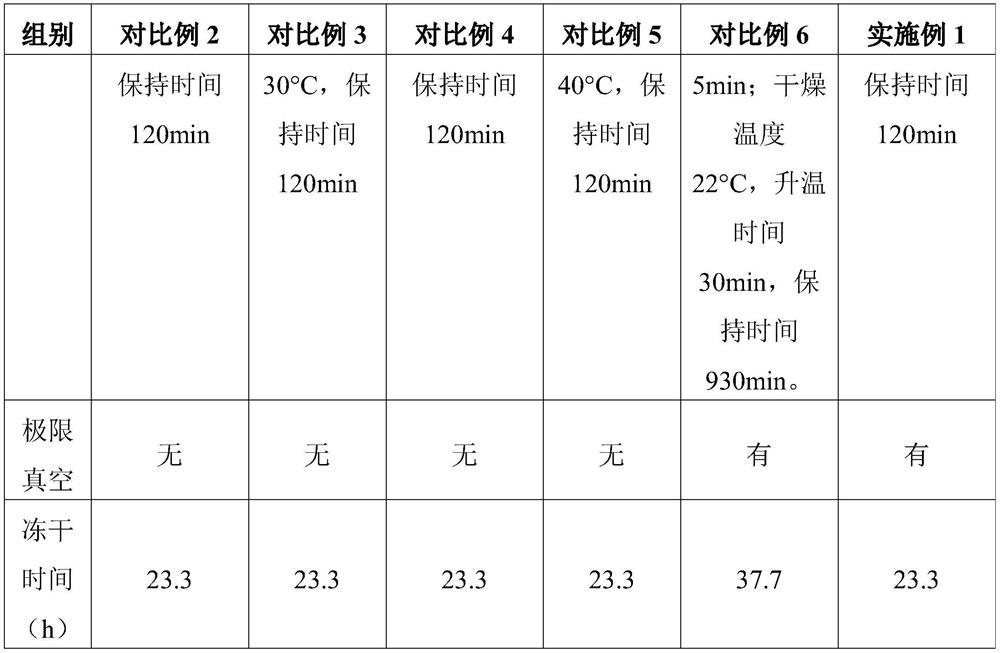
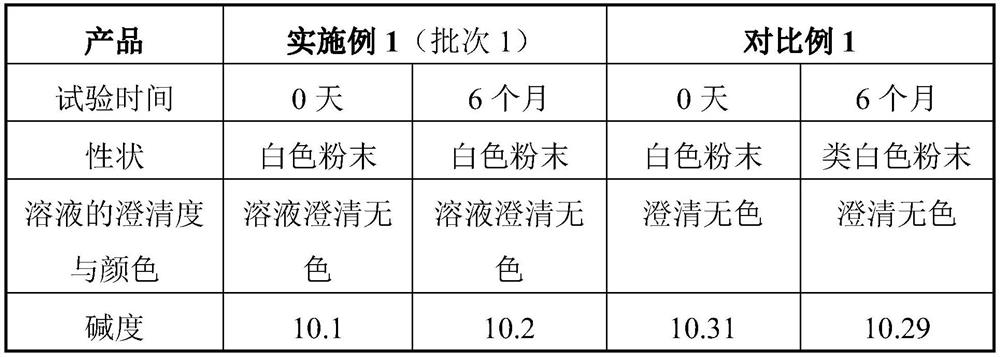
Embodiment 1
[0031] The preparation of embodiment 1 injection pantoprazole sodium freeze-dried preparation
[0032] In Example 1, batches 1, 2 and 3 are different batches of products prepared by the method in Example 1.
[0033] The prescription of this product is as follows Table 1:
[0034] Table 1 Product prescription
[0035] Raw materials unit dose Pantoprazole Sodium Sesquihydrate 45.1mg Edetate Disodium (for injection) 1mg sodium hydroxide 0.24mg Water for Injection Add to 2ml
[0036] Take the water for injection below 30°C, inject nitrogen gas, add water for injection according to the amount of 95% of the water for injection in the prescription amount, cool the water for injection below 30°C, fill with nitrogen gas, and weigh edetate disodium according to the prescription amount Put it into the liquid mixing tank, and circulate and stir for at least 5 minutes until it is completely dissolved; add a part of sodium hydroxide solution, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com