Application of liriope spicata saponin B in preparation of medicine for treating skin inflammation
A technology of ryeposide saponin and skin inflammation, applied in the field of application of ryeposide saponin B in the preparation of medicines for treating skin inflammation, can solve problems such as the application of ryeposide saponin B that has not been reported in the literature, and achieve good protection effect, reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
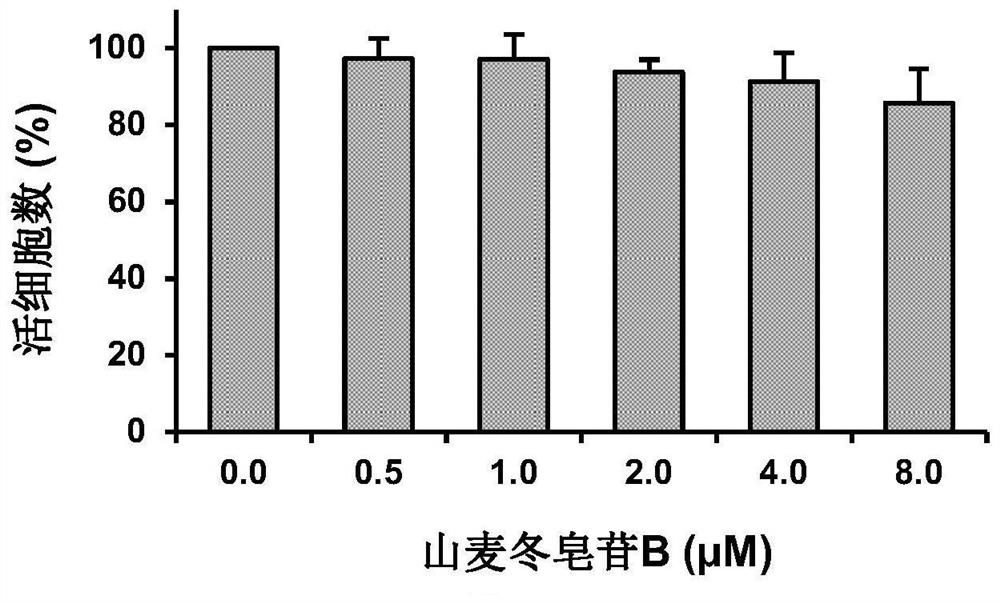
[0031] The effect of the different concentrations of ryeposide B on the activity of HaCaT cells in Example 1
[0032] (1) Experimental method
[0033] HaCaT keratinocytes (2×10 4 Cells / ml) were seeded in 35mm culture dishes and placed in 5% CO 2 , and cultured in a 37°C incubator for 24 hours to allow the cells to attach to the culture dish. Set the concentration of ryeposide B to 0.5 μM, 1.0 μM, 2.0 μM, 4.0 μM, 8.0 μM, and treat the cells for 48 hours. Digest HaCaT cells from the culture dish with trypsin, add DMEM medium containing 10% heat-inactivated FBS and antibiotics (100 units / mL penicillin, 100 μg / mL streptomycin) to make cell suspension, press 80 μL After the cell suspension was mixed with 20 μL of trypan blue solution (0.04 g / mL) for 2 minutes, the number of viable cells after treatment was measured with a hemocytometer under a light microscope. Blue cells are considered dead cells, while unstained cells are considered live cells. Cell viability=(total number o...
Embodiment 2
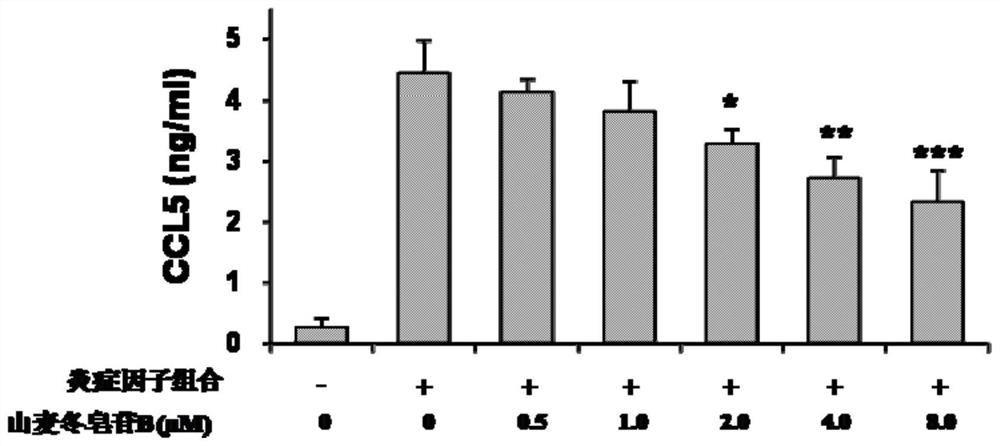
[0036] Example 2 The effect of ryeposide B on the level of CCL5 in cells treated with inflammatory cytokine composition
[0037] (1) Experimental method
[0038] HaCaT keratinocytes (1×10 6 cells / ml) were seeded in 6-well plates and placed in 5% CO 2, and cultured in a 37°C incubator for 24 hours to allow the cells to attach to the culture dish. HaCaT cells were stimulated with a combination of inflammatory cytokines interleukin-6 (15 ng / mL), interleukin-1β (10 ng / mL) and tumor necrosis factor-α (10 ng / mL), and the concentration of ryeposide B was set to 0.5 μM , 1.0 μM, 2.0 μM, 4.0 μM, 8.0 μM, the cells were treated for 24 hours. According to the instructions of the enzyme-linked immunosorbent assay kit, the level of CCL5 in the culture supernatant was determined by using it.
[0039] (2) Experimental results
[0040] like figure 2 As shown, ryeposide B at 0.5uM had no effect on CCL5 levels. At 1.0 uM, ryeposide B had some effect on CCL5, but did not reach statistical...
Embodiment 3
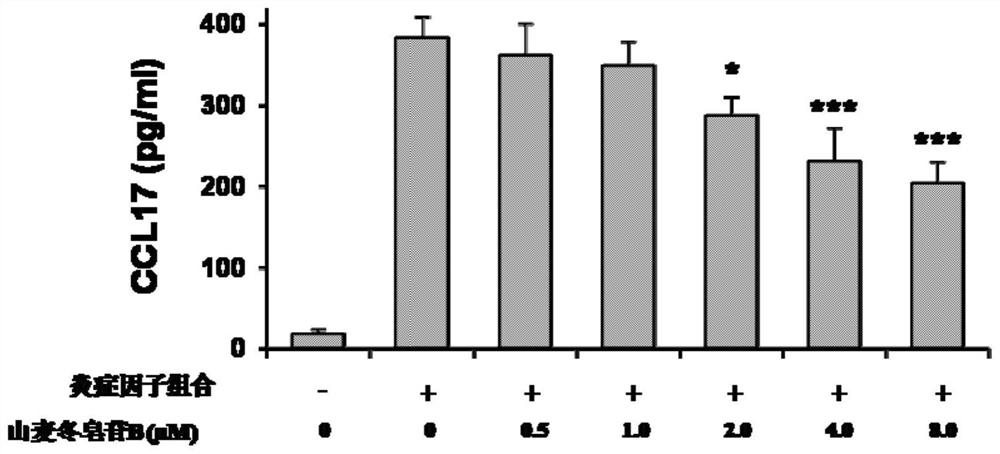
[0042] Example 3 Effects of Limeposide B on CCL17 Levels in Cells Treated with Inflammatory Cytokine Composition
[0043] (1) Experimental method
[0044] HaCaT keratinocytes (1×10 6 cells / ml) were seeded in 6-well plates and placed in 5% CO 2 , and cultured in a 37°C incubator for 24 hours to allow the cells to attach to the culture dish. Stimulate HaCaT cells with a combination of inflammatory cytokines interleukin-6 (15 ng / mL), interleukin-1β (10 ng / mL) and tumor necrosis factor-α (10 ng / mL), and set the concentration of ryeposide B to 0.5 μM, 1.0 μM, 2.0 μM, 4.0 μM, 8.0 μM, cells were treated for 24 hours. According to the instructions of the enzyme-linked immunosorbent assay kit, the level of CCL17 in the culture supernatant was determined by using it.
[0045] (2) Experimental results
[0046] like image 3 As shown, low doses of ryeposide B (0.5 and 1.0 μM) did not significantly reduce the level of CCL17, 2.0 μM of ryeposide B moderately decreased the level of CCL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com