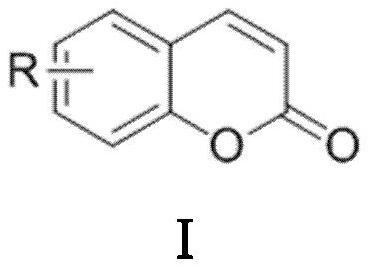
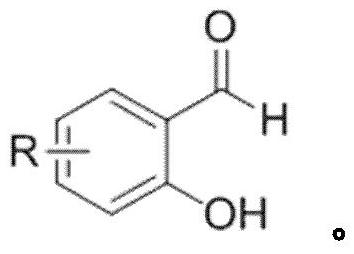
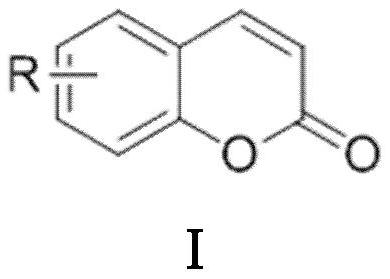
Synthesis method of coumarin derivatives
A technology of coumarins and a synthesis method is applied in the field of synthesizing coumarin derivatives, can solve the problems of long time, need to stir the reaction overnight, or even react for several days, achieves less catalyst usage, improves production efficiency, The effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: a kind of synthetic method of coumarin derivatives:
[0028] Add 0.6mmol of PPA and 0.3mL of solvent DMF into the round bottom flask, then add 0.3mmol of salicylaldehyde and 1.2mmol of acetic anhydride in sequence, and stir in an oil bath at 145°C under nitrogen protection for 3h; Wash the organic layer with water and saturated sodium chloride solution, then dry and wash the organic layer with anhydrous sodium sulfate, filter, and the residue after the filtrate is concentrated under reduced pressure is subjected to silica gel column chromatography (V 石油醚 / V 乙酸乙酯 =50:1) separation and purification to obtain coumarin derivative I-1 with a yield of 91%.
[0029] The nuclear magnetic resonance (NMR) of the prepared coumarin derivatives (I-1) 1 H NMR and 13 C NMR) detection data are: 1 HNMR (600MHz, CDCl 3 )δ7.73(d,J=9.5Hz,1H),7.58–7.53(m,1H),7.51(dd,J=7.7,1.4Hz,1H),7.36(d,J=8.3Hz,1H), 7.32–7.28(m,1H),6.45(d,J=9.5Hz,1H). 13 C NMR (151MHz, CDCl 3 )δ160.8...
Embodiment 2
[0030] Embodiment 2: a kind of synthetic method of coumarin derivatives:
[0031] Add 0.6mmol of PPA and 0.3mL of solvent DMF into the flask, then add 0.3mmol of 4-fluorosalicylaldehyde and 1.2mmol of acetic anhydride in sequence, and stir in an oil bath at 145°C for 4h under nitrogen protection; after the reaction, extract with ethyl acetate twice, Wash the organic layer with water and saturated sodium chloride solution successively, then dry and wash the organic layer with anhydrous sodium sulfate, filter, and the residue after the filtrate is concentrated under reduced pressure is subjected to silica gel column chromatography (V 石油醚 / V 乙酸乙酯 =40:1) separation and purification to obtain coumarin derivative I-2 with a yield of 80%.
[0032] The nuclear magnetic resonance (NMR) of the prepared coumarin derivatives (I-2) 1 H NMR and 13 C NMR) detection data are: 1 HNMR (600MHz, CDCl 3 )δ7.71(d, J=9.6Hz, 1H), 7.50(dd, J=8.5, 6.0Hz, 1H), 7.10–7.01(m, 2H), 6.40(d, J=9.6Hz, 1H)...
Embodiment 3
[0033] Embodiment 3: a kind of synthetic method of coumarin derivatives:
[0034] Add 0.6mmol of PPA and 0.3mL of solvent DMF into the flask, then add 0.3mmol of 5-chlorosalicylaldehyde and 1.2mmol of acetic anhydride in sequence, and stir in an oil bath at 145°C under nitrogen protection for 3h; after the reaction, extract with ethyl acetate twice, Wash the organic layer with water and saturated sodium chloride solution successively, then dry and wash the organic layer with anhydrous sodium sulfate, filter, and the residue after the filtrate is concentrated under reduced pressure is subjected to silica gel column chromatography (V 石油醚 / V 乙酸乙酯 =30:1) separation and purification to obtain coumarin derivative I-3 with a yield of 82%.
[0035] The nuclear magnetic resonance (NMR) of the prepared coumarin derivatives (I-3) 1 H NMR and 13 C NMR) detection data are: 1 HNMR (600MHz, DMSO) δ8.00(d, J=9.6Hz, 1H), 7.82(d, J=2.5Hz, 1H), 7.63(dd, J=8.8, 2.6Hz, 1H), 7.42(d, J=8.8Hz, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com