Aurovertin B derivative and preparation method and application thereof
A derivative and reaction technology, applied in the field of medicine, can solve problems such as lack of targeted standard treatment plans, and achieve the effects of improving bioavailability, good safety, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
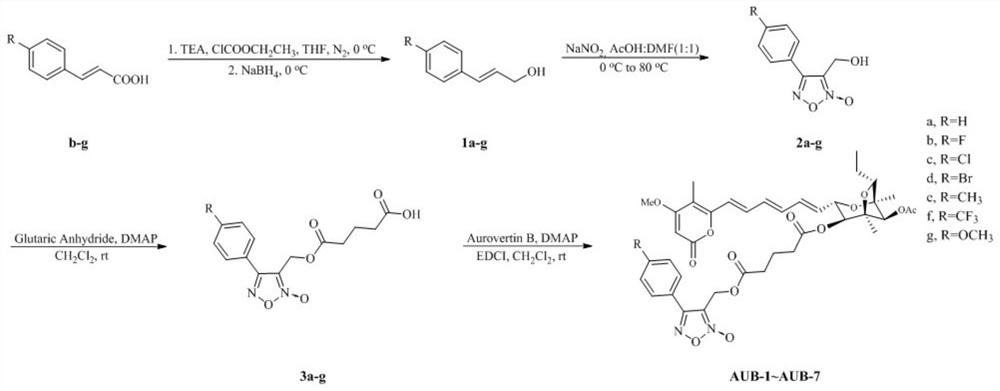
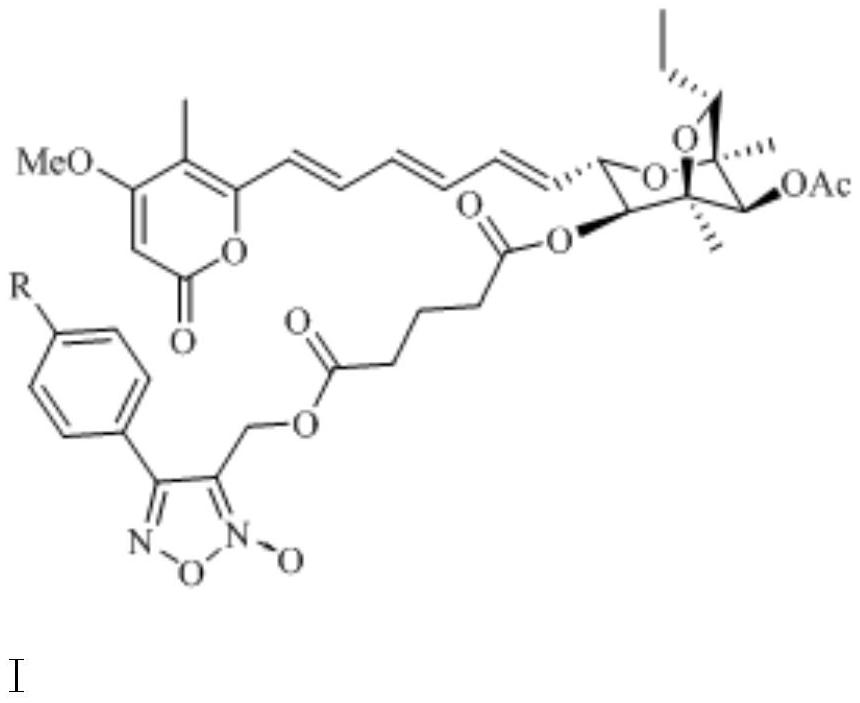
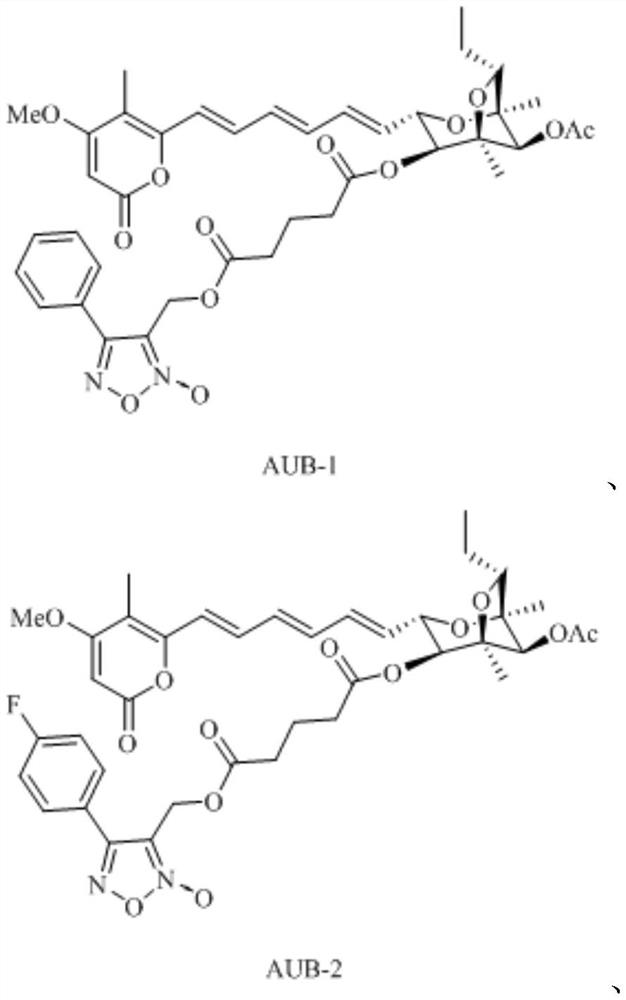
[0026] Embodiment 1: the synthesis of aurovertin B derivative AUB-1 (synthetic route diagram such as figure 1 shown)
[0027]
[0028] Take cinnamic acid (1mmol), in N 2 Add anhydrous THF in the atmosphere, slowly add triethylamine (1mmol) dropwise at 0°C; after the reaction, slowly add ClCOOCH dropwise 2 CH 3 (1mmol); white precipitate appeared in the reaction solution, filtered, and NaBH was added in batches to the filtrate under stirring at 0°C 4 Reaction (4mmol) to obtain a reaction mixture; after the reaction is completed, slowly add methanol to the reaction mixture to quench, overnight at room temperature; adjust the pH of the reaction mixture to acidity, add purified water, and use CH 2 Cl 2 Extract, add anhydrous sodium sulfate to the enriched organic layer and spin dry, perform silica gel column chromatography separation, the eluent is petroleum ether: acetone = 7:1 or chloroform: methanol = 100:1, and concentrate and dry under reduced pressure to obtain compou...
Embodiment 2
[0033] Embodiment 2: the synthesis of aurovertin B derivative AUB-2 (synthetic route figure such as figure 1 shown)
[0034]
[0035] Get p-fluorocinnamic acid (1.0g, 6.2mmol) in two-necked flask, under N 2 10 mL of anhydrous THF was added in the atmosphere, and triethylamine (0.63 g, 6.2 mmol) was slowly added dropwise at 0°C over 2 min. After reacting for 5 minutes, slowly add ClCOOCH dropwise 2 CH 3 (0.67g, 6.2mmol) over 5min. White precipitate appeared in the reaction solution, filtered, and NaBH was added to the filtrate in batches under stirring at 0°C 4 (2.35g, 62.1mmol), react for more than 30min. After the reaction was completed, 10 mL of methanol was slowly added to quench it, and reacted overnight at room temperature. Adjust the pH of the reaction solution to acidic with concentrated hydrochloric acid, add 10 mL of pure water, and 2 Cl 2 (3×10mL) was extracted three times, the enriched organic layer was added with anhydrous sodium sulfate and spin-dried, ...
Embodiment 3
[0040] Embodiment 3: the synthesis of aurovertin B derivative AUB-3 (synthetic route figure such as figure 1 shown)
[0041]
[0042] Get p-chlorocinnamic acid (1.0g, 5.5mmol) in two-necked flask, under N 2 10 mL of anhydrous THF was added in the atmosphere, and triethylamine (0.56 g, 5.5 mmol) was slowly added dropwise at 0°C over 2 min. After reacting for 5 minutes, slowly add ClCOOCH dropwise 2 CH 3 (0.59g, 5.5mmol) over 5min. White precipitate appeared in the reaction solution, filtered, and NaBH was added to the filtrate in batches under stirring at 0°C 4 (0.79g, 20.8mmol), react for more than 30min. After the reaction was completed, 10 mL of methanol was slowly added to quench it, and reacted overnight at room temperature. Adjust the pH of the reaction solution to acidic with concentrated hydrochloric acid, add 10 mL of pure water, and 2 Cl 2 (3×10mL) was extracted three times, the enriched organic layer was added with anhydrous sodium sulfate and spin-dried, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com