Sensitizer drug, pharmaceutical composition and application
A technology of sensitizers and drugs, applied in the field of biomedicine, can solve the problems of lack of specificity, effective cell permeability, etc., and achieve the effect of improving sensitivity, enhancing sensitivity, and wide targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
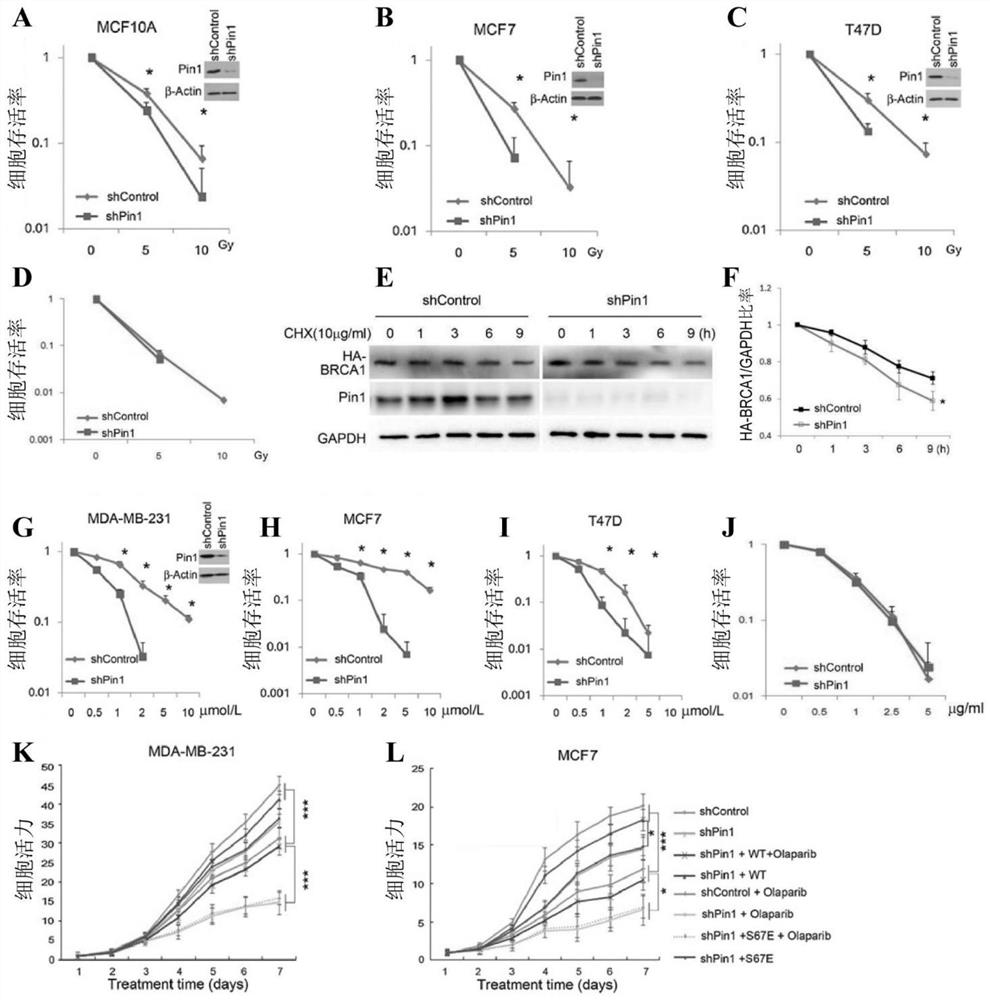
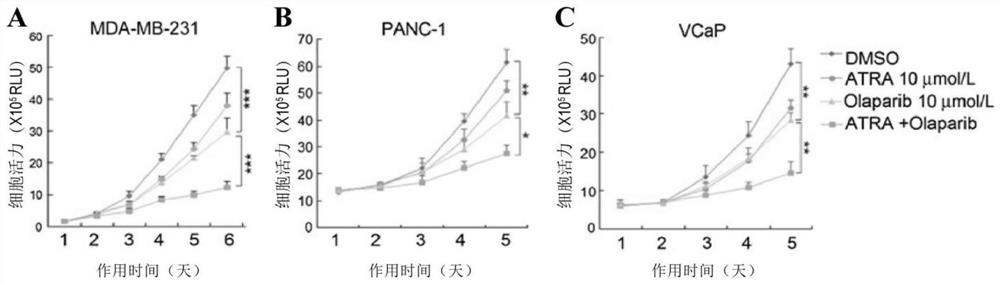
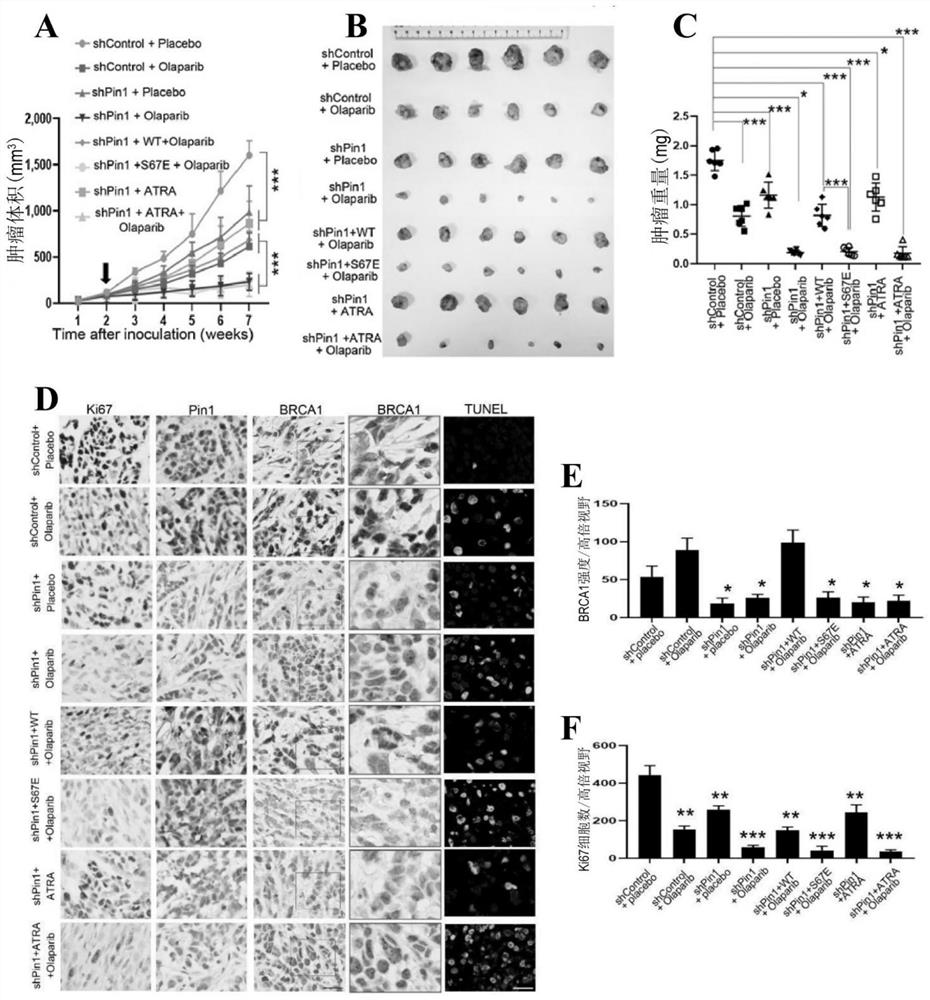
[0053] In vitro and in vivo biological tests
[0054] (1) Materials and methods
[0055] (1) Drugs
[0056]ATRA (Sigma) was dissolved in 150 mM DMSO and diluted in 1:1 PBS and 1M NaOH working solution and administered intraperitoneally in 200 ml at a dose of 1.5 mg / kg once a day. Olaparib (Selleckchem) was formulated and used as described in the literature Cancer Discov. 2012; 2: 1048-63.
[0057] (2) Cells and cell culture
[0058] MCF10A cells derived from ATCC were cultured in DMEM / F-12, adding 5% horse serum, 20ng / ml epidermal growth factor (EGF), 0.5μg / ml hydrocortisone, 100ng / ml cholera toxin, 10μg / ml insulin, Culture was as described in the literature Methods.2003; 30: 256-68. ATCC-derived MCF-7, T47D, MDA-MB 231, AU565, HEK293 were cultured in DMEM containing 10% FBS. SUM149 was cultured in Ham's F-12 medium containing 5% bovine serum (FBS), insulin (5 μg / mL) and hydrocortisone (2 μg / mL).
[0059] Choose the radiation dose according to the experiment and the cell...
Embodiment 2
[0088] Pharmaceutical composition containing all-trans retinoic acid 10 mg and olaparib 100 mg
[0089] 10 grams of all-trans retinoic acid and 100 grams of olaparib were crushed through a 120 mesh sieve respectively, and then mixed with 25 grams of croscarmellose sodium, 20 grams of mannitol, and 15 grams of polysaccharides crossed through an 80 mesh sieve. Vitone was mixed evenly, and 30 grams of 10% starch slurry was added to make soft materials, granulated through a 24-mesh sieve, then ventilated and dried at 50 degrees Celsius, and after granulation with a 20-mesh sieve, mixed with 2 grams of colloidal silicon dioxide and 2 2.5 grams of sodium stearyl fumarate is mixed uniformly, and compressed into tablets to obtain pharmaceutical composition tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com