Lithium secondary battery
A lithium secondary battery and electrolyte technology, which is applied in secondary batteries, lithium batteries, battery electrodes, etc., can solve the problems of lithium ion influence, solvent decomposition, and inability to insert lithium ions, etc., and achieve the effect of excellent cycle characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
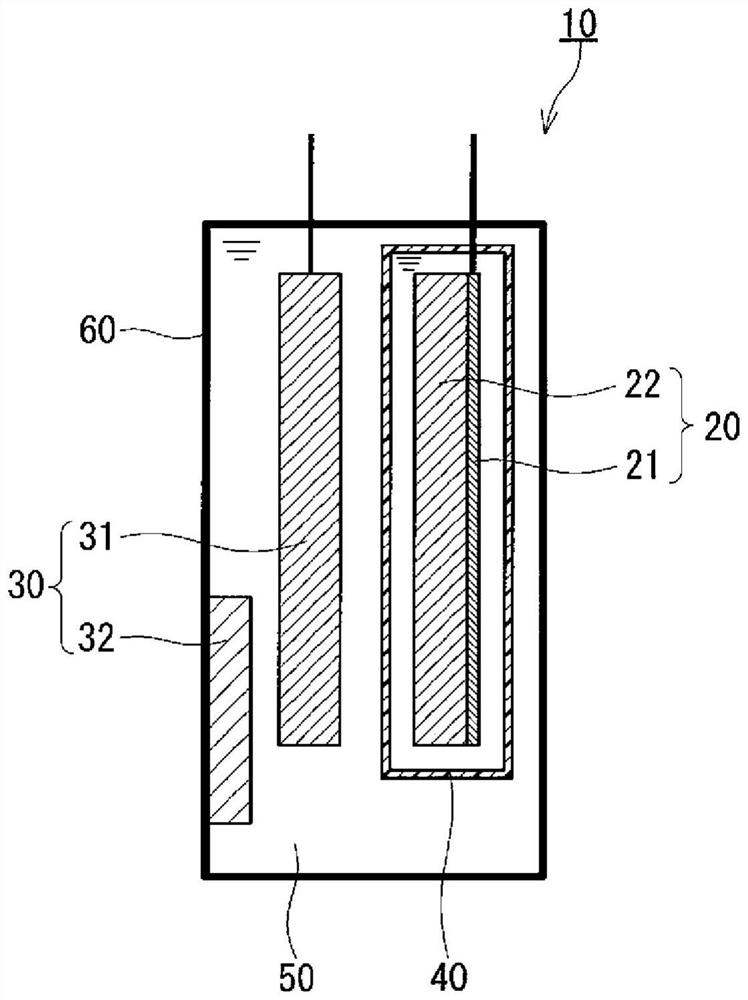
[0059] figure 1 A cross section of a lithium secondary battery according to an embodiment of the present disclosure is shown. Lithium secondary battery 10 includes positive electrode 20 , negative electrode 30 , and electrolytic solution 50 . The electrolytic solution 50 contains a solvent and a negative electrode mediator, and is in contact with the positive electrode 20 and the negative electrode 30 . A negative electrode medium is dissolved in the solvent of the electrolytic solution 50 . The negative electrode 30 has a negative electrode current collector 31 and a negative electrode active material 32 . The oxidation-reduction reaction of the negative electrode active material 32 in the negative electrode 30 proceeds through the negative electrode mediator.
[0060] The lithium secondary battery 10 further includes a container 60 . Container 60 is sealed. The container 60 is made of insulating and corrosion-resistant material. The positive electrode 20 , the negative...
Embodiment 1
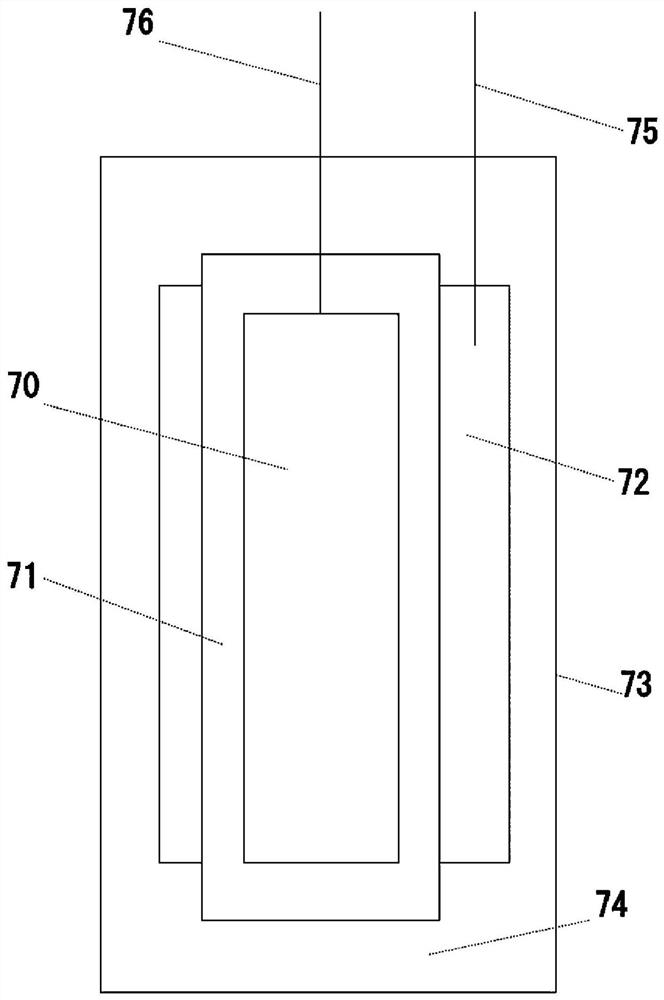
[0197] A 2×2 cm copper foil (a) was covered with a polypropylene microporous separator (b), and the whole was covered with a large amount of lithium metal foil (c). Next, tabs are respectively installed on the copper foil and the lithium metal to form an electrode group. Then, it was housed in a laminated case (d), and after injecting an ether solution (e) in which biphenyl was dissolved at 0.1 mol / L, the opening of the laminated case was thermally welded and sealed to produce figure 2 Shown is a battery cell for potentiometric measurements.
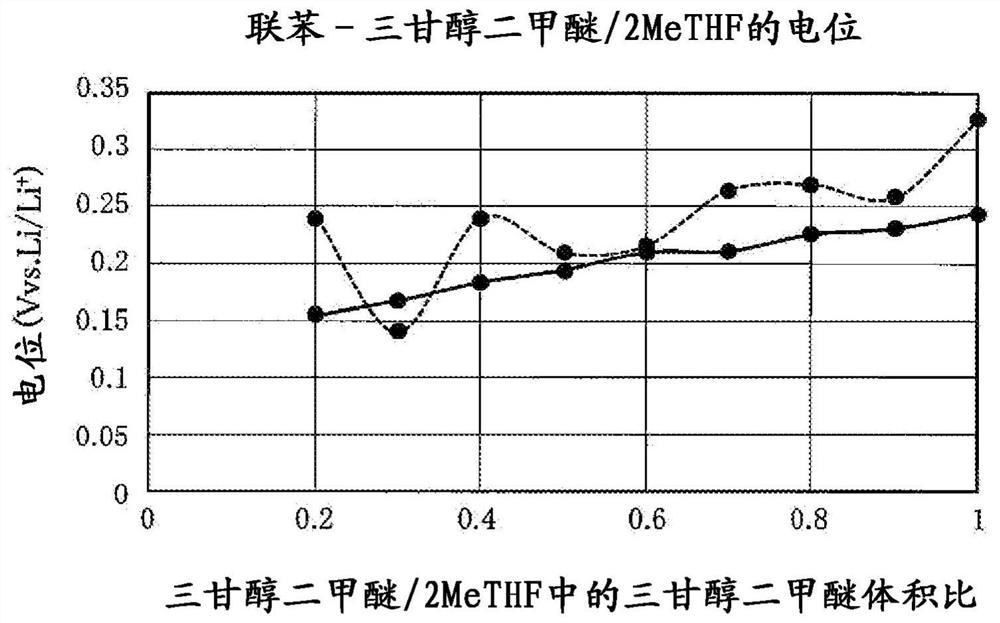
[0198] The solvent of the ether solution contains 2methyltetrahydrofuran as a cyclic ether, and triglyme as glyme. The ratio of the volume of triglyme to the volume of the solvent of the ether solution was 0.2. In other words, the ratio of the volume of 2-methyltetrahydrofuran to the volume of triglyme was 8:2. In the ether solution, 1mol / L LiPF was dissolved as a supporting salt 6 . As described above, the battery cell 1 for poten...
Embodiment 2
[0203] Prepared LiFePO containing 8mAh equivalent as positive electrode active material 4 , 2 mAh equivalent Al foil as the negative electrode active material, rough surfaced copper foil as the negative electrode current collector, lithium salt as the electrolyte, and 2-methyltetrahydrofuran solution of biphenyl. made with figure 1 Lithium secondary battery shown. The positive electrode is covered with a separator made of a microporous membrane. LiPF is used as lithium salt 6 . LiPF in electrolyte 6 The concentration is 1mol / L. The concentration of biphenyl in the electrolyte solution was adjusted to 0.00625 mol / L, 0.0125 mol / L, 0.025 mol / L or 0.05 mol / L to obtain 4 lithium secondary batteries.
[0204] A charge-discharge test of the obtained lithium secondary battery was performed. A charge-discharge test was performed under the conditions of a charge-discharge current of 1 mA, a charge time of 2 hours, and a cut-off voltage of 2 V during discharge. The results are sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com