Compound solid compositions containing phenylephrine bitartrate and aspirin, preparation and application thereof
A technology of phenylephrine and solid composition, which is applied in the direction of medical preparations containing active ingredients, drug combinations, anti-inflammatory agents, etc., and can solve problems such as unreasonable composition, degradation of active ingredients, and discoloration of liquid medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
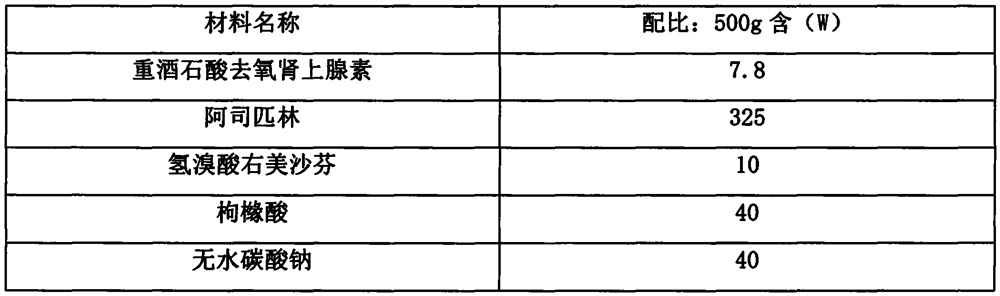
[0049] prescription
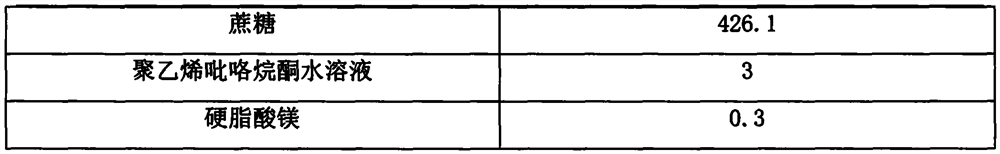
[0050]
[0051]
[0052] Preparation method:
[0053] 1. Mixing of active ingredients: Take the prescribed amount of aspirin and dextromethorphan hydrobromide and mix evenly, then add phenylephrine tartrate in equal increments while stirring, after stirring evenly, keep away from light and airtight to obtain active ingredients Ingredient mix powder, set aside;
[0054] 2. Preparation of acid granules: take half of the above mixed powder, add the prescribed amount of citric acid and sucrose and mix evenly, add part of polyvinylpyrrolidone aqueous solution to make soft material, granulate to obtain acid granules, and set aside;
[0055] 3. Preparation of alkali granules: Take half of the above mixed powder, add anhydrous sodium carbonate of the prescribed amount, mix well, add the remaining polyvinylpyrrolidone aqueous solution to make a soft material, granulate to obtain alkali granules, and set aside;
[0056] 4. Mix the above-mentioned acid granu...
Embodiment 2
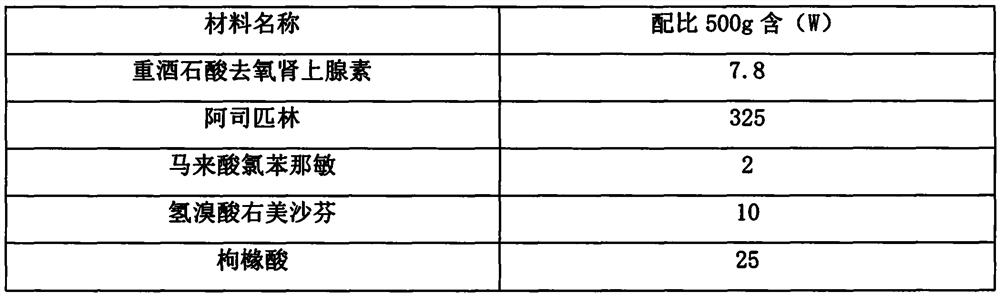
[0058] prescription:
[0059] material name Proportion: 500g contains (W) Phenylephrine Bitartrate 7.8 aspirin 325 Chlorpheniramine maleate 2 citric acid 50 Anhydrous Sodium Carbonate 50 dextrin 62 Hydroxypropylmethylcellulose 3 Micropowder silica gel 0.2
[0060] Preparation method:
[0061] 1. Mixing of active ingredients: After taking the prescribed amount of aspirin and chlorpheniramine maleate and mixing them evenly, add phenylephrine tartrate in equal increments under stirring, after stirring evenly, keep away from light and airtight to obtain Active ingredient mixed powder, set aside;
[0062] 2. Preparation of acid granules: Take one-half of the mixed powder of the above active ingredients, add the prescribed amount of citric acid and dextrin and mix evenly, add part of the soft material made of hydroxypropyl methylcellulose, and granulate to obtain acid Granules, spare;
[0063] 3. Preparation of alkali...
Embodiment 3
[0066] prescription:
[0067] material name Proportion 1000g contains (W) Phenylephrine Bitartrate 7.8 aspirin 500 Dextromethorphan Hydrobromide 10 doxylamine succinate 6.25 citric acid 50 Anhydrous Sodium Carbonate 50 sucrose 372.75 Aqueous solution of polyvinylpyrrolidone 3 Micropowder silica gel 0.2
[0068] Preparation method:
[0069] 1. Mixing of active ingredients: take the prescribed amount of aspirin, dextromethorphan hydrobromide, and doxylamine succinate and mix evenly, then add phenylephrine tartrate in equal increments while stirring, and mix well , protected from light, airtight, to obtain active ingredient mixed powder, and set aside;
[0070] 2. Preparation of acid granules: Take half of the mixed powder of the above active ingredients, add the prescribed amount of citric acid and sucrose and mix evenly, add part of polyvinylpyrrolidone aqueous solution to make soft material, granulate to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com