Human II-type thrombocytopenia iPS cell line and preparation method and application thereof
A thrombocytopenia and cell line technology, applied in the biological field, can solve the problems of difficulty in obtaining megakaryocyte samples, short lifespan, and obstacles to megakaryocyte maturation, and achieve the effect of convenient sample source and long lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The technical solutions in the embodiments of the present invention will be clearly and completely described below in conjunction with the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only a part of the embodiments of the present invention, rather than all the embodiments. Based on the embodiments of the present invention, all other embodiments obtained by those of ordinary skill in the art without creative work shall fall within the protection scope of the present invention.
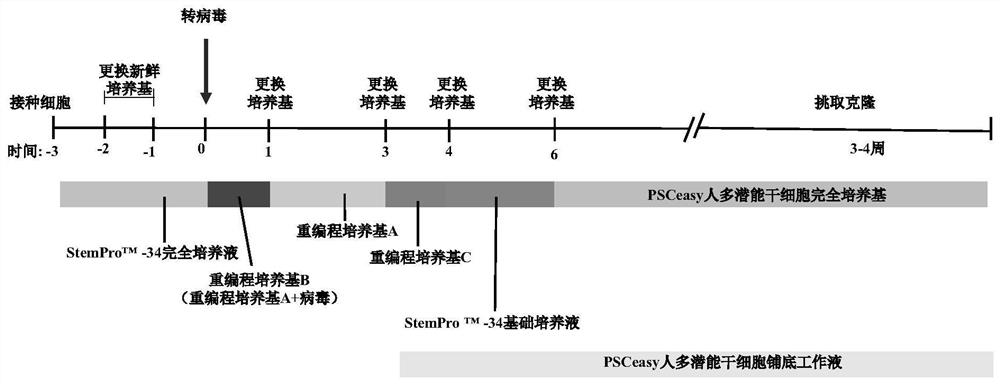
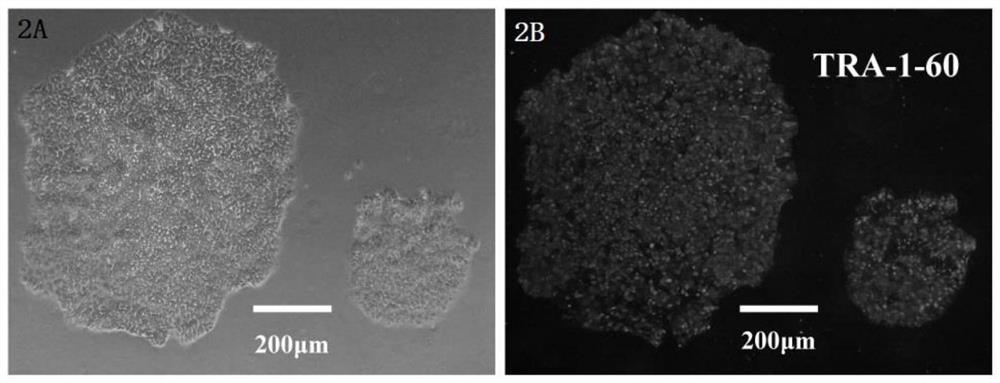
[0029] The present invention transfers virus vectors carrying specific transcription factors into type II thrombocytopenia patients bone marrow-derived CD34 + Cells to produce iPS cell lines, the entire operation process is as attached figure 1 Shown. Specific steps are as follows:
[0030] 1. CD34 from the bone marrow of the target patient + Cell acquisition
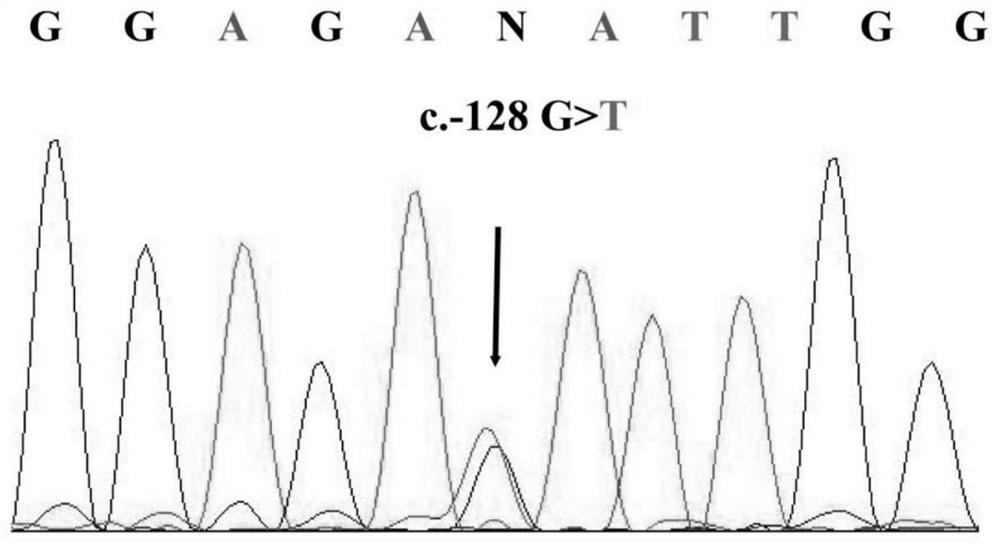
[0031] Collect the 5'-UTR region of Ankerd26 gene c.-128G> T heterozygous mutati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com