Heterostructure iron/cobalt bimetallic phthalocyanine electrocatalyst and preparation method and application thereof
A heterostructure, bimetallic technology, applied in the field of electrochemical energy storage, can solve the problems of general oxygen reduction reaction catalytic performance, uneven microscopic morphology, large iron phthalocyanine particles, etc., and achieve excellent electrochemical performance and electrical conductivity. The effect of improved, high kinetic current density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
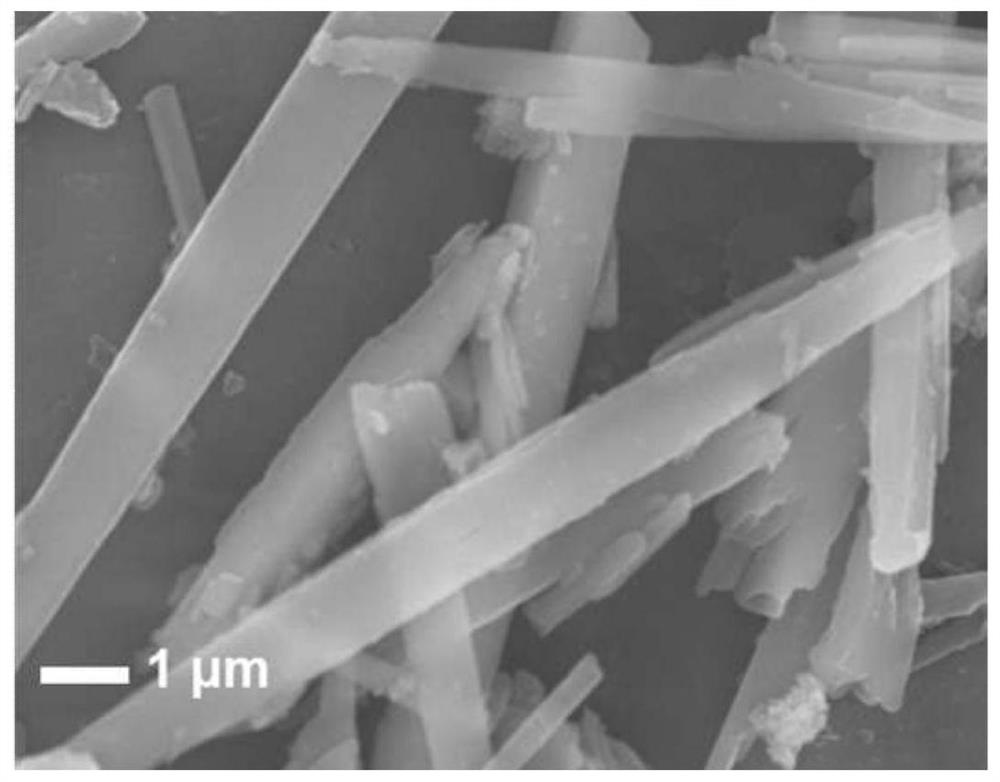
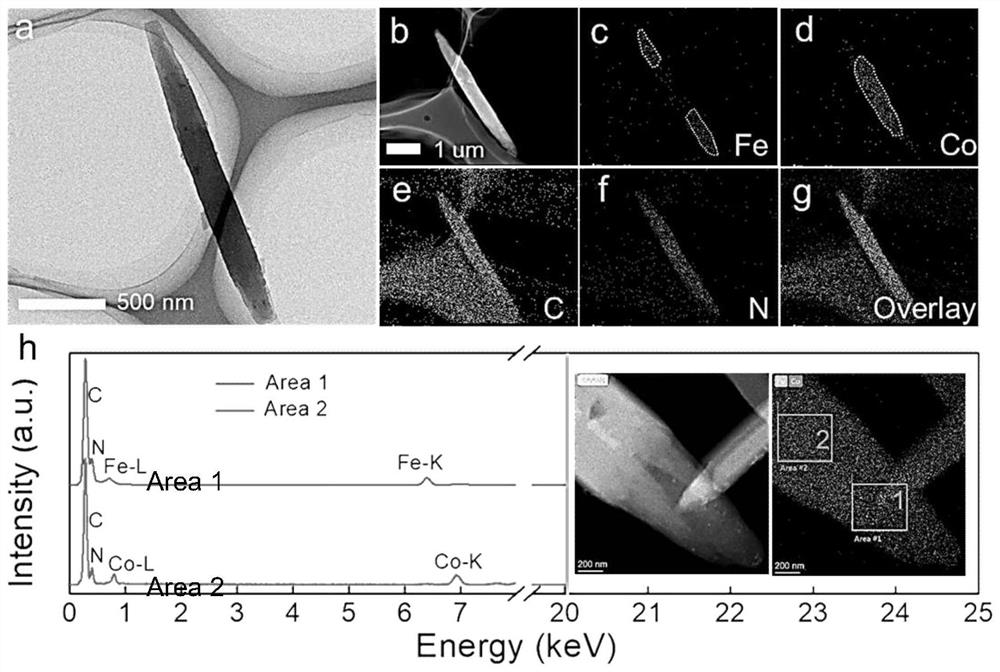
Image
Examples
Embodiment 1
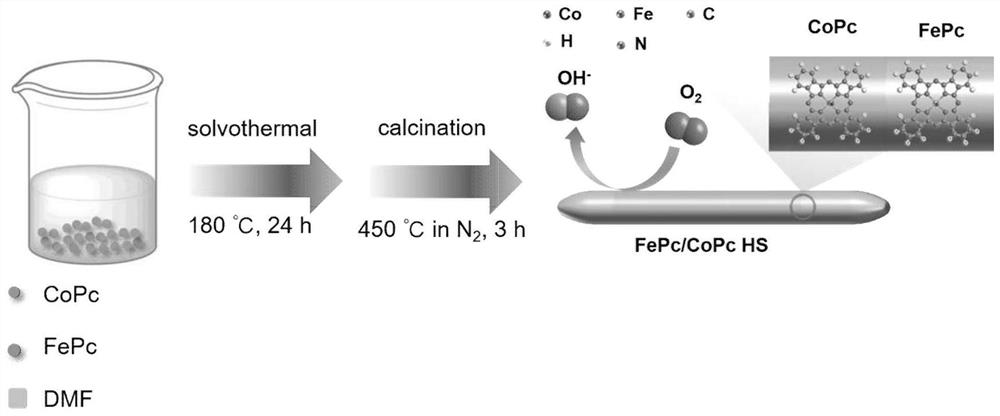
[0031] 1) Add low crystallinity cobalt phthalocyanine (0.150g) and iron phthalocyanine (0.050g) powder into 70mL N,N-dimethylformamide solvent and stir for 30 minutes, so that the powder is evenly dissolved in the solvent to obtain purple black solution;
[0032] 2) transfer the stirred solution into a reaction kettle, and conduct a solvothermal reaction at 180° C. for 24 hours;
[0033] 3) After the reaction is over, take out the solution after the reactor is cooled, wash it with alcohol three times, and dry it in vacuum at 80°C to obtain the precursor of the bimetallic phthalocyanine;
[0034] 4) The above product was calcined in an inert gas tube furnace at 450° C. for 3 hours at a low temperature to obtain a heterostructured iron / cobalt bimetallic phthalocyanine (FePc / CoPc HS) catalyst.
[0035] Taking the heterostructured iron / cobalt double metal phthalocyanine catalyst obtained in this example as an example, its synthesis schematic diagram is shown in the attached figu...
Embodiment 3
[0051] 1) Add commercial low-crystallinity cobalt phthalocyanine (0.100g) and iron phthalocyanine (0.100g) powder into 70mL N,N-dimethylformamide solvent and stir for 30 minutes to dissolve the powder evenly in the solvent , to obtain a purple-black solution;
[0052] 2) Transfer the stirred solution into a reaction kettle, and conduct a solvothermal reaction at 180°C for 24 hours;
[0053] 3) After the reaction is over, take out the solution after the reactor is cooled, wash it with alcohol three times, and dry it in vacuum at 80°C to obtain the precursor of the bimetallic phthalocyanine;
[0054] 4) The above product was calcined in an inert gas tube furnace at 450°C for 3 hours at a low temperature to obtain a heterostructured iron / cobalt bimetallic phthalocyanine (1:1) catalyst.
[0055] Taking the heterostructure iron / cobalt bimetallic phthalocyanine obtained in this example as an example to carry out ORR catalytic test, as shown in the attached Figure 6 As shown in a,...
Embodiment 4
[0057] 1) Add commercial low-crystallinity cobalt phthalocyanine (0.050g) and iron phthalocyanine (0.150g) powder into 70mL N,N-dimethylformamide solvent and stir for 30 minutes to dissolve the powder evenly in the solvent , to obtain a purple-black solution;
[0058] 2) Transfer the stirred solution into a reaction kettle, and conduct a solvothermal reaction at 180°C for 24 hours;
[0059] 3) After the reaction is over, take out the solution after the reactor is cooled, wash it with alcohol three times, and dry it in vacuum at 80°C to obtain the precursor of the bimetallic phthalocyanine;
[0060] 4) The above product was calcined in an inert gas tube furnace at 450° C. for 3 hours at a low temperature to obtain a heterostructured iron / cobalt bimetallic phthalocyanine (3:1) catalyst.
[0061] Taking the heterostructure iron / cobalt bimetallic phthalocyanine obtained in this example as an example to carry out ORR catalytic test, as shown in the attached Figure 6 As shown in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com