Linezolid dry-mixing suspension and preparation method thereof
A technology of linezolid and dry suspension, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of reducing product safety and instability, and achieve reduction The use of excipients, increasing the number of applicable people, improving the effect of taste and safety issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
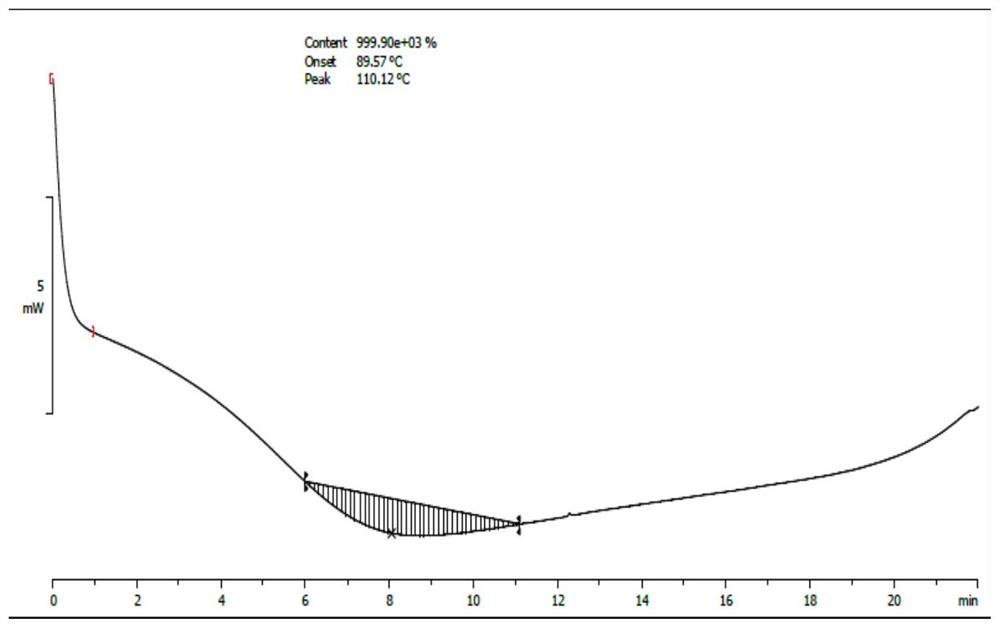
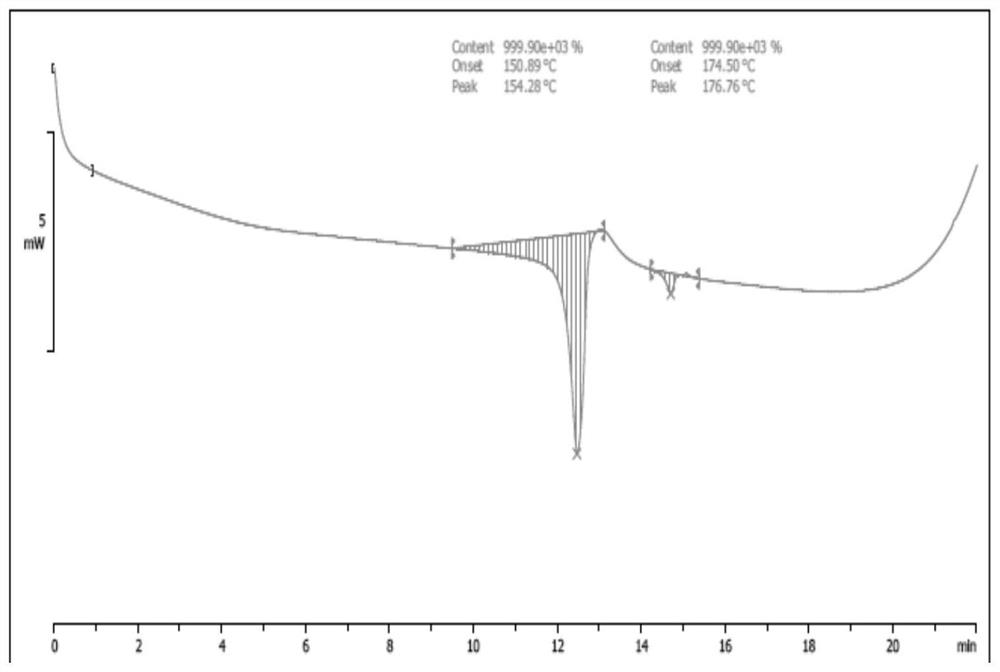
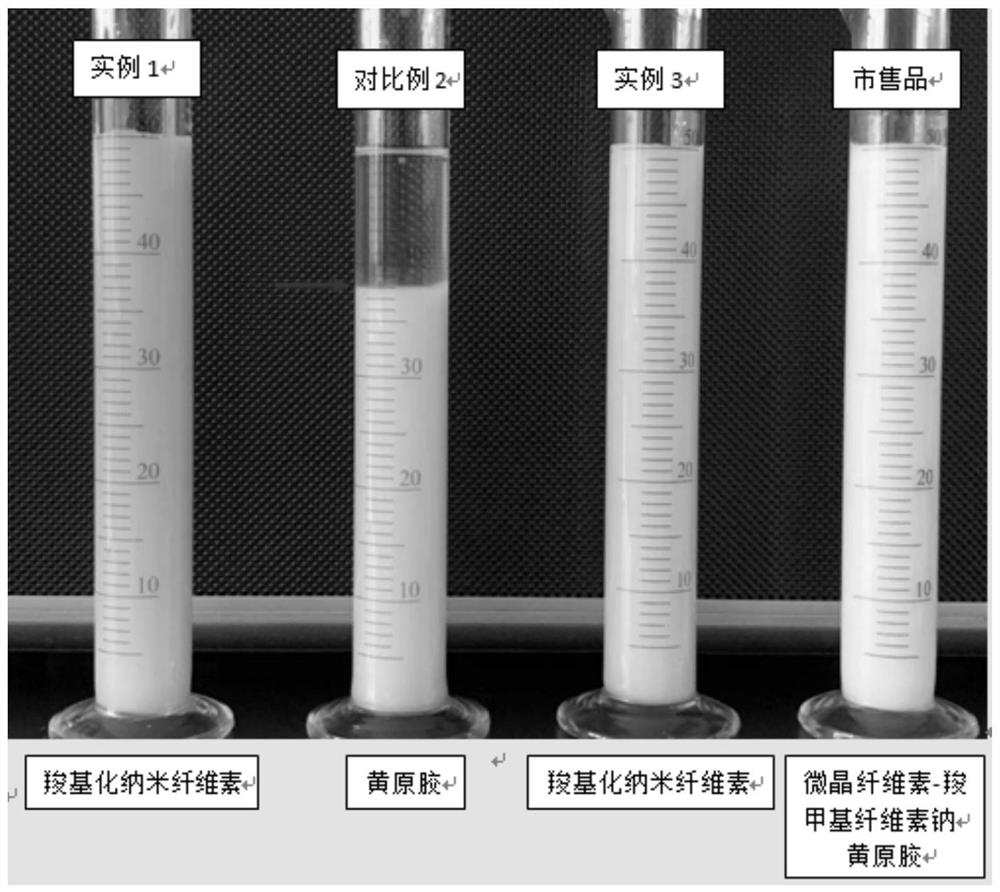
Image
Examples
example 1
[0061]
[0062]
[0063] Process:
[0064] (1) Prepare materials according to the prescription of Example 1, and pass 80 mesh sieves of mannitol for subsequent use.
[0065] (2) Add linezolid, mannitol, carboxylated nanocellulose, citric acid, and sodium citrate into a wet granulator and mix for 5 minutes to obtain a mixture. Then 35g of water was added into the wet granulator through an atomizer, and stirred for 60s to prepare a soft material. Pass the soft material through a 30-mesh sieve and granulate to obtain drug-containing granules.
[0066] (3) Put the drug-containing granules into a vacuum drying oven to dry for 4 hours at a temperature of 60° C., and use a 30-mesh sieve to sieve the granules.
[0067] (4) Add the drug-containing granules together with sodium benzoate, orange flavor and silicon dioxide into a multi-sport mixer, mix for 20 minutes, and mix well.
example 3
[0076]
[0077] According to the above formula, the same preparation method as in Example 1 was adopted to obtain the finished product 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com