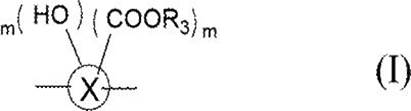Diamine compound, heat resistant resin or heat resistant resin precursor using same, photosensitive resin composition, cured film, and display device
A heat-resistant resin, amine compound technology, applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, photosensitive materials for optomechanical equipment, etc. Reduce heat resistance and other problems, to achieve the effect of improving heat resistance, increasing cross-linking density, and reducing outgassing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Preparation method of structural formula A
[0046] The synthesis of the binary amine compound shown in the formula (1) can be produced based on the method for producing known diamine compounds; in the formula (1), in particular, the present invention, the present invention EXAMPLES Select the use of nitro compound precursors in palladium carbon (PD / C) or Lani nickel to catalyzes hydrogen reduction; then hydrolyzing the ester group in the acid solution, can be mentioned as an example, showing The following reduction reactions (as shown in the reaction formula B and C).
[0047]
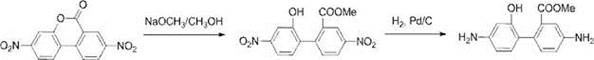
[0048] Reactive B
[0049] The specific reaction conditions of the reaction B are as follows: a solvent is made of methanol, and 2,7-dinitropyl-3, 4 benzoxin is dissolved therein, and the mixture is added to 10% 10 wt. % PD / C, in-house hydrogen, at 20-50 ° C, reaction 5-48 h. Then, PD / C was removed, and the concentrated concentrated filtrate was rotated.
[0050]
[0051] Reactive C
[005...
Synthetic example 1
[0135] Synthesis of 4,4'-diaminosome-2-hydroxy-2'-biphenyl carbonate (A)
[0136]
[0137] Under dry nitrogen gas protection, 2,7-dinitropyl-3, 4 benzoxin (20 g, 69.9 mmol) was dissolved in 300 ml of tetrahydrofuran (THF), and 80 g of reduction iron powder was added, and 20 ml 10 wt was added dropwise. % Hydrochloric acid, warmed to 80 ° C, stirring reaction for 24 h, adjusting pH to weak acidity with sodium carbonate, ethyl acetate extraction, water was washed 3 times, separated from organic phases, concentrated, recrystallized 4,4'-diamino-2 Hydroxy-2'-Benzoic acid (A).
Synthetic example 2
[0139] Synthesis of 4,4'-di (4-amine-based phenyloxy) -2-hydroxy-2'-carboxybenzene compound (b)
[0140]
[0141] Under dry nitrogen stream, 2,7-dihydroxy-3,4 benzoxin (20 g, 87.6 mmol) was dissolved in 300 ml N, N-dimethylpyrrolidone (NMP), and 4-fluorine - Nitrobenzene (24.7 g, 175.3 mmol), potassium carbonate (48.5 g, 350.6 mmol), 50 ° C reaction 10 h, poured into water, ethyl acetate extraction, concentrated organic phase, to obtain a nitro compound.
[0142]
[0143] The resulting 2,7-b (4-nitrophenyloxy) -3, 4 benzoxin was dissolved in 500 mL of ethanol, add 80 g of zinc shavitation, dripping 20 ml 10 wt% hydrochloric acid, warming up to 50 ° C, stirring reaction 24 h, filtered off Pd / C, concentrated filtrate, recrystallized 2,7-di (4-aminophenyloxy) -2-hydroxy-2'-carboxybenzene compound (b) .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com