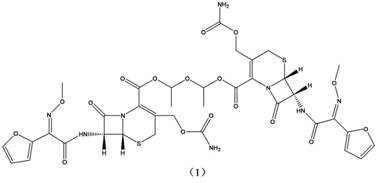
Method for synthesizing cefuroxime axetil dimer
A technology of furoxetin axetil dimer and cefuroxime, which is applied in the field of synthesis of cefuroxime axetil dimer, can solve the problems of difficulty in establishing quality control methods, difficulty in obtaining impurity reference substances, and low impurity content, etc. To achieve the effect of ensuring the safety of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
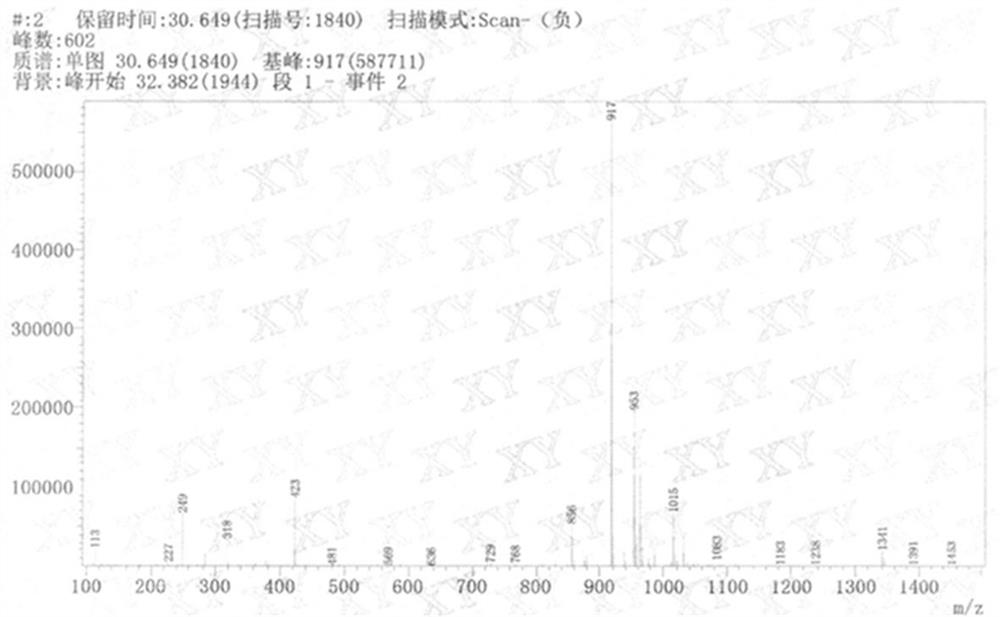
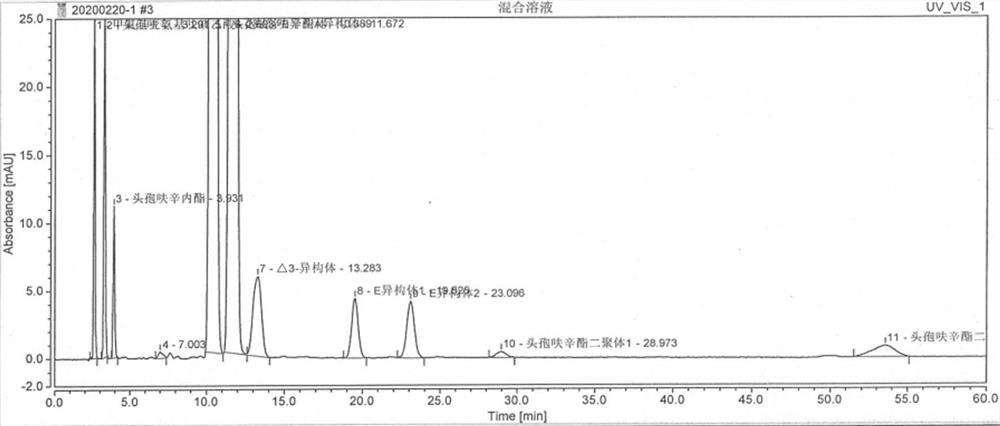
Image
Examples
Embodiment 1
[0017] Add paraldehyde (158.6g, 1.2mol) and tetrahydrofuran (500ml) into the reaction flask, add trimethylbromosilane (183.7g, 1.2mol) dropwise, after the addition is complete, stir and heat to reflux for 5 hours; to room temperature, add triethylamine (100ml) and cefuroxime (42.4g, 1.0mol), then raise the temperature to reflux, stir the reaction for 2-12h, cool to room temperature, concentrate under reduced pressure, successively use 10% sodium sulfite solution, 10% Wash with sodium carbonate solution and purified water, dry over anhydrous sodium sulfate, and concentrate at 35-40°C to obtain a yellow oil. Recrystallization from acetone gave 68.8 g of cefuroxime axetil dimer, with a yield of 75%.
Embodiment 2
[0019] Add paraldehyde (79.3g, 0.6mol) and diethyl ether (500ml) into the reaction flask, add trimethylbromosilane (91.8g, 0.6mol) dropwise, after the addition is complete, stir and heat to reflux for 6 hours; To room temperature, add triethylamine (50ml) and cefuroxime (21.2g, 0.5mol), then raise the temperature to reflux, stir and react for 8h, cool to room temperature, concentrate under reduced pressure, use 10% sodium sulfite solution, 10% sodium carbonate successively The solution was washed with purified water, dried over anhydrous sodium sulfate, and concentrated at 25-40°C to obtain a yellow oil. Recrystallization from acetone gave 30.95 g of cefuroxime axetil dimer, with a yield of 67.5%.
Embodiment 3
[0021] Add paraldehyde (79.3g, 0.6mol) and tetrahydrofuran (500ml) into the reaction flask, add trimethylbromosilane (91.8g, 0.6mol) dropwise, after the addition is complete, stir and heat to reflux for 5 hours; To room temperature, add potassium carbonate (150g) and cefuroxime (21.2g, 0.5mol), then raise the temperature to reflux, stir and react for 10h, cool to room temperature, concentrate under reduced pressure, use 10% sodium sulfite solution, 10% sodium carbonate solution successively , washed with purified water, dried over anhydrous sodium sulfate, and concentrated at 25-40°C to obtain a yellow oil. Recrystallization from acetone gave 26.3 g of cefuroxime axetil dimer, with a yield of 57.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com