Anti-H5N1 virus endocytosis antibody PTD-7B and application thereof
A PTD-7B, virus technology, applied in the direction of antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve the problem of being located on the inside of the virus envelope
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Construction, expression and purification of M1 protein recombinant expression plasmid pET-SUMO-M1 of H5N1 virus M1 protein
[0030] Design and synthesize primers P1 and P2 for M1:
[0031] P1: 5'-atgagtcttctaaccgaggtc-3'; its base sequence is shown in SEQ ID NO.1 in the sequence table.
[0032] P2: 5'-CCg gaattc ttaCttgaatcgctgcatctgcact-3'; its base sequence is shown in SEQID NO.2 of the sequence table.
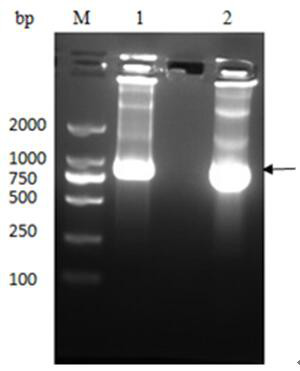
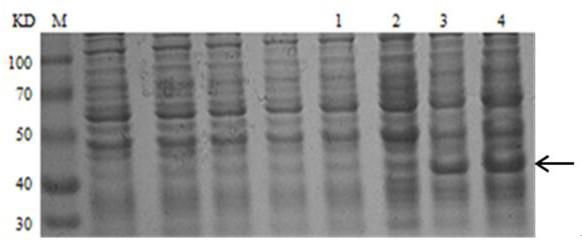
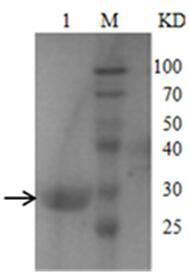
[0033] The M1 protein gene was amplified by PCR using H5N1 cDNA as a template, and cloned into the PET-SUMO vector to construct the plasmid pET-SUMO-M1, which was then transferred into T-shot competent cells and carried out on an agar plate containing kanamycin resistance. initial screening. A single colony was selected and cultured in LB liquid medium; the plasmid was extracted with a plasmid recovery kit and identified by PCR. The product was analyzed by 1% agarose gel electrophoresis, and a band of about 750 bp was obtained, which was consistent with...
Embodiment 2
[0036] Example 2 Screening of phage single-chain antibody library
[0037] Inject all the frozen Tomlinson I and J libraries into 200 mL 2×TY medium (containing 100 µg / mL Amp and 1% glucose), and culture with shaking at 37°C until the OD600 value is about 0.4, from Take out 50 mL bacterial liquid from the culture medium, add 2×10 11 The helper phage KM13 was placed in a water bath at 37°C for 30 minutes, centrifuged at 3000×g for 10 minutes at 4°C, and the pellet was resuspended with 50 mL of 2×TY medium (containing 100 µg / mL Amp, 50 µg / mL Kan and 0.1% glucose). Suspended and cultured overnight at 30°C with shaking. Centrifuge the overnight product at 4°C, 3500×g for 30 min, collect 40 mL of the supernatant, add 10 mL of ice-cold PEG / NaCl solution (final concentration is 20% PEG-6000, 2.5 mol / L NaCl), mix well and store on ice Place for more than 1 h, centrifuge at 4°C, 3500×g for 30 min, discard the PEG / NaCl solution, resuspend the pellet in 2 mL of PBS, centrifuge at 11600...
Embodiment 3
[0038] Example 3 Screening of anti-M1-scFv
[0039] Coat the purified M1 protein on a 96-well microtiter plate overnight at 4°C. Discard the supernatant the next day, block with 2% Milk-PBS at 37°C for 2 hours, add the prepared secondary phage antibody library, incubate with vigorous shaking at room temperature for 60 minutes, discard the liquid after standing for 60 minutes, and replace with PBS containing 0.1% Twenn-20 Wash 10 times, after washing, gently pat dry the remaining liquid in each well, add 50 µL eluent (5 mg / mL trypsin-PBS) to each well, shake vigorously at room temperature for 10 min, elute phage, collect and store at 4 °C ;
[0040] Infect E.coli TG1 with the eluted phage, spread on TYE plates (containing 100 μg / mL ampicillin and 1% glucose) and culture overnight at 37°C. Use the helper phage KM13 to amplify the phage library, and recover the phage by PEG / NaCl; repeat the above process 3 times, a total of 4 rounds of screening;
[0041] Phage infection after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com