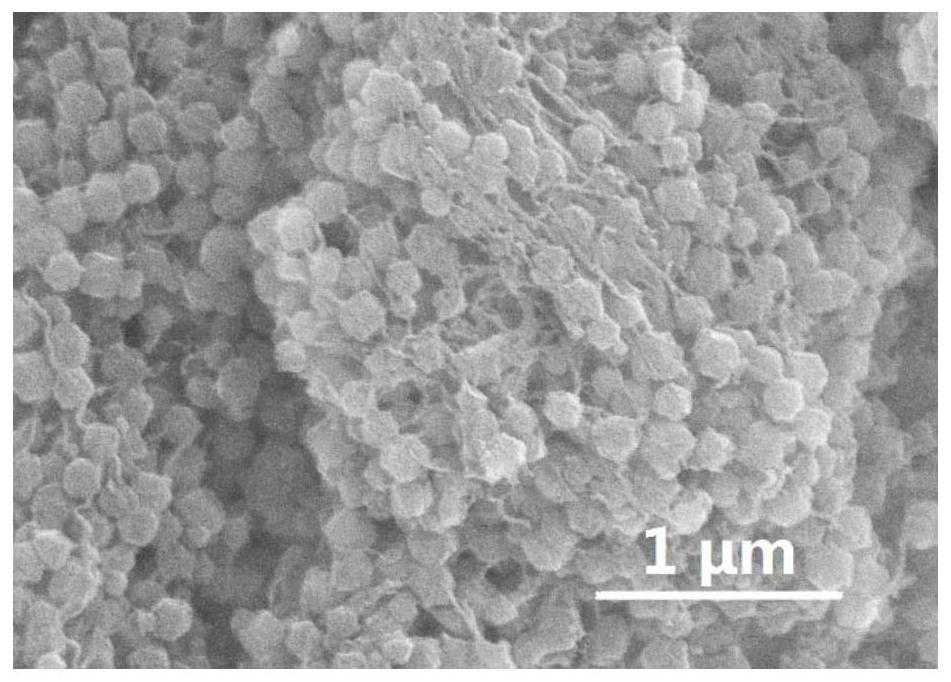
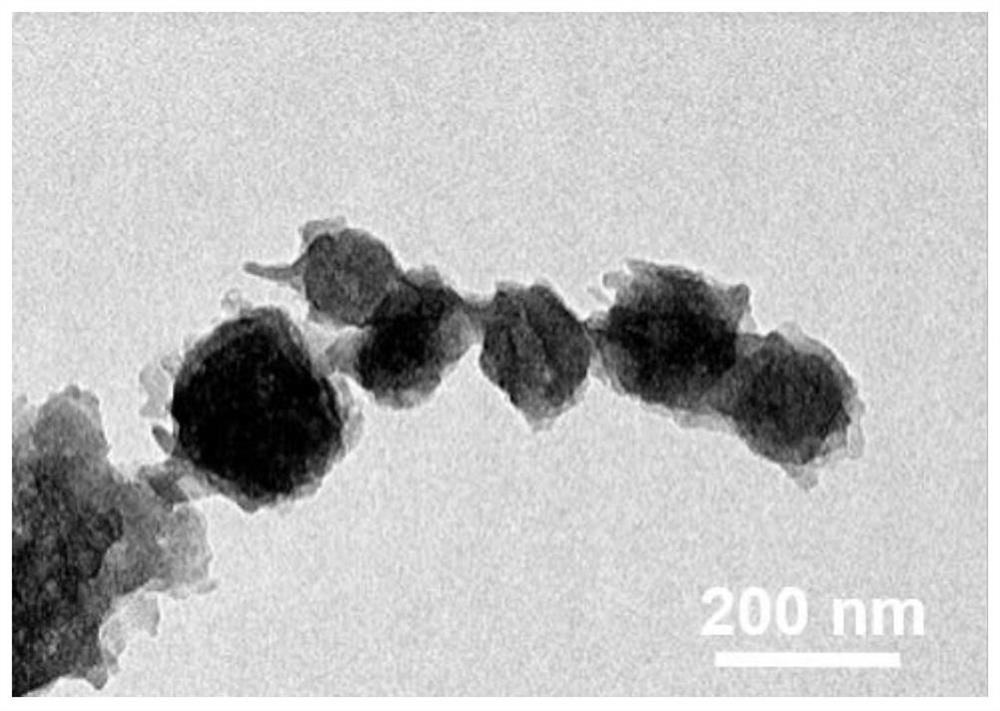
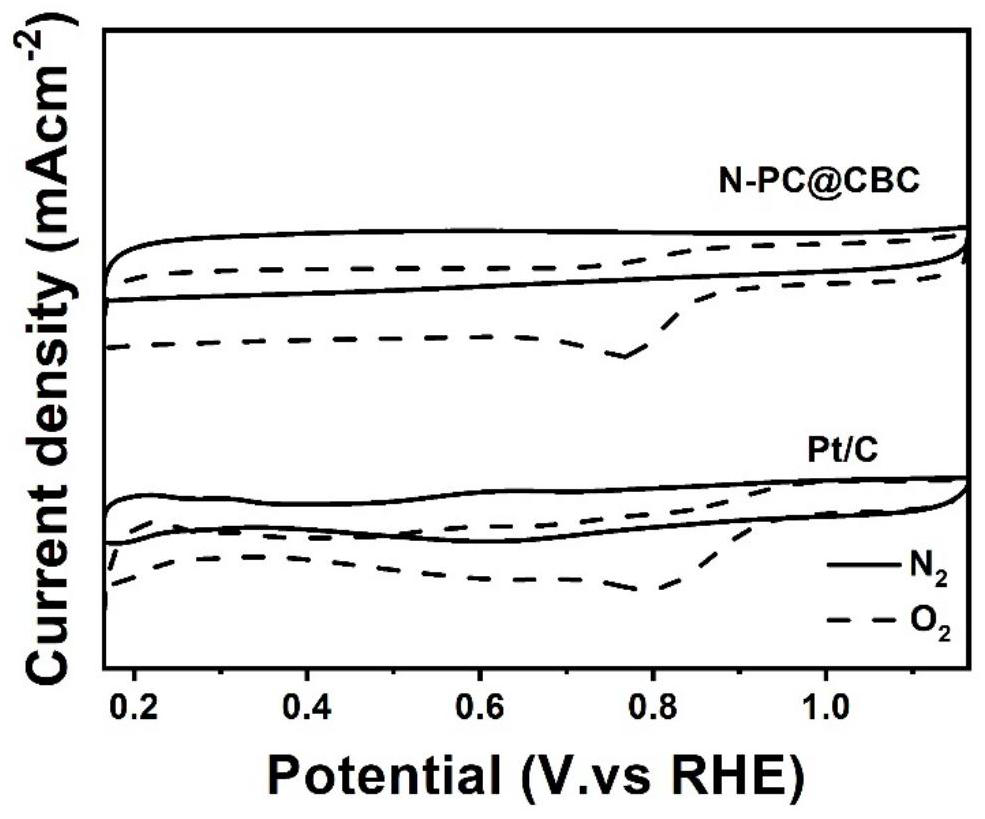
Preparation method and application of electro-catalytic oxygen reduction catalytic material N-PC @CBC
A catalytic material and electrocatalytic technology, applied in the direction of electrochemical generators, circuits, electrical components, etc., to achieve large surface area, excellent ORR performance, and ensure the effect of surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of material N-PC@CBC:
[0037] (1) Disperse 0.1 g of BC and 4.75 mmol of zinc nitrate hexahydrate in 100 mL of methanol. After stirring for 24 hours, the well-dispersed mixture was added to a solution of 2-methylimidazole dissolved in 100 mL of 20 mmol in methanol.
[0038] (2) The mixture was stirred for 2 h and allowed to stand at room temperature for 24 h to form the ZIF-8@BC nanofiber composite, and the resulting material was freeze-dried.
[0039] (3) Carbonize the dried material in a tube furnace under an Ar atmosphere. The temperature programming steps are as follows: the temperature is 2 °C min -1 The rate was increased from room temperature to 800 °C, and then held at 800 °C for 2 hours. Thereafter, first at 2°C min -1 Decrease the temperature to 600°C at a rate of 5°C min -1 rate down to room temperature. The product N-PC@CBC was obtained. The obtained product has the best ORR catalytic activity, at 0.1mol -1 / L of KOH solution, ...
Embodiment 2
[0040] Embodiment 2: in the step (3) in the embodiment 1, the temperature programming step is: the temperature is 2 ℃ min -1 The rate was increased from room temperature to 900 °C, and then held at 900 °C for 2 hours. Thereafter, first at 2°C min -1 Decrease the temperature to 900°C at a rate of 5°C min -1 rate down to room temperature. Other steps are with embodiment 1. The ORR catalytic performance of the obtained product: at 0.1mol -1 / L of KOH solution, the onset potential is 0.814V, and the half-wave potential is 0.755V.
Embodiment 3
[0041]Embodiment 3: The material obtained in Embodiment 1 is made into an electrode by a film coating method, which is used for the negative electrode of a sodium ion battery. The material ratio is obtained material: conductive substance: binder = 8:1:1. The performance of coin-type batteries assembled with electrodes made of the obtained materials was evaluated by CV and galvanostatic charge / discharge tests. After the first few cycles, the CV curves gradually overlap in subsequent cycles, indicating good reversibility during Na ion storage. The initial charge and discharge capacities of the fabricated batteries were 348 and 620 mAh g, respectively. -1 , corresponding to an initial Coulombic efficiency of 56.1%. The Coulombic efficiency gradually increases in the first few cycles and maintains a high value close to 100% in subsequent cycles. The battery made at 0.1A g -1 exhibited good cycling stability at low current densities and achieved 200mAh g after 400 cycles -1 Ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com