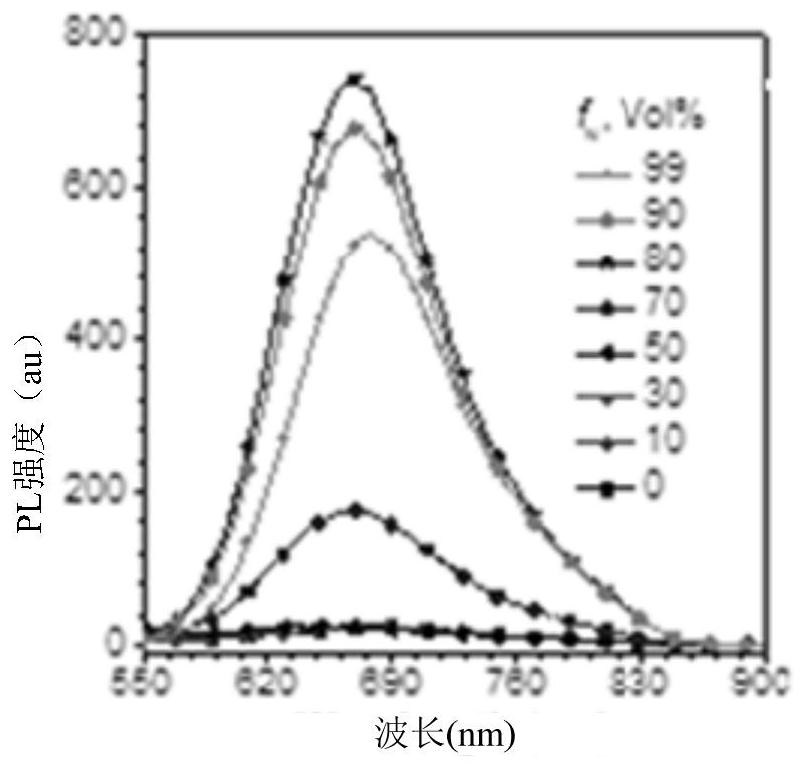
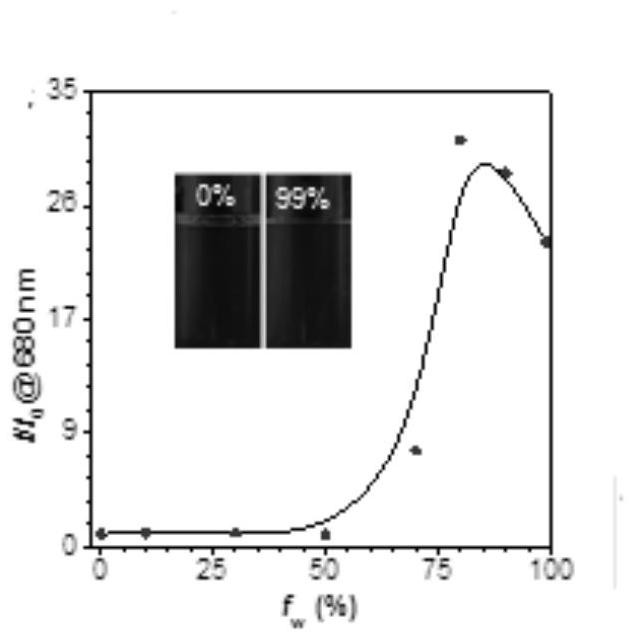
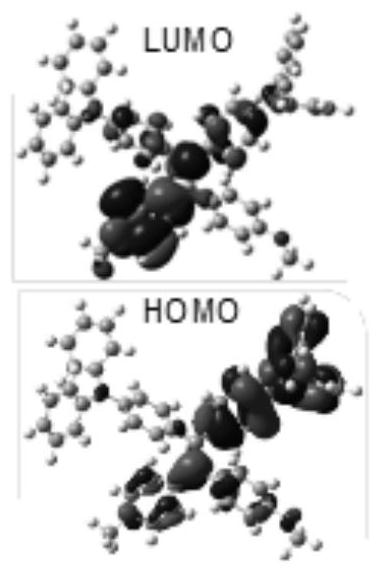
Corannulene-incorporated AIE nanodots with highly suppressed nonradiative decay for boosted cancer phototheranostics in vivo
A technology of sulfene, a therapeutic agent, applied to AIE nanodots wrapped with sulfene with high non-radiative attenuation inhibition for enhancing the field of in vivo cancer photodiagnosis, which can solve the problems of undisclosed and optimized fluorescence and ROS generation capacity of AIE spots.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Synthesis of compound 2
[0127] Compound 2: At -78 ℃, TiCl 4 (1mL, 9.0mmol) was slowly added to a suspension of Zn powder (1.17g, 18.0mmol) in anhydrous THF (50mL). After refluxing for 2 h, a mixture of compound 1 (2.325 g, 4.5 mmol) and 4-methoxyphenyl-4-pyridyl ketone (0.640 g, 3 mmol) in anhydrous THF (20 mL) was added to the reaction. The mixture continued to reflux for 5 hours. After removing the solvent under reduced pressure, the residue was extracted with DCM and washed with anhydrous Na 2 SO 4 dry. The crude product was purified by silica gel column chromatography using DCM as eluent. Compound 2 is a yellow solid with a yield of 70%. 1 H NMR (400MHz, CD 2 Cl 2 ,δ): 8.33 (d, 2H, J = 0.011), 7.27-7.22 (m, 8H), 7.05-7.03 (m, 9H), 7.02-7.01 (m, 2H), 7.00-6.99 (m, 1H) , 6.97 (d, 2H, J = 0.013), 6.95 (d, 2H, J = 0.011), 6.93 (d, 2H, J = 0.004), 6.92 (d, 2H, J = 0.007), 6.82 (d, 2H ,0.004), 6.79(d,2H,0.005), 6.71(d,2H,J=0.021), 3.77(s,3H); 13 C NMR(100MHz, CD 2 Cl ...
Embodiment 2
[0129] Synthesis of TPP-TPA
[0130] Compound 2 (0.174g, 0.25mmol) was dissolved in 20mL of toluene, and then 0.1mL of CH was added 3 I (excess) to form a mixture. The reaction mixture was refluxed overnight. After cooling to room temperature, the precipitate was filtered and washed three times with cold toluene. The obtained solid was dissolved in 20 mL of acetone and 100 mg of KPF was added 6 Perform ion exchange for 2h. The solvent was removed and the solid was washed with water. The pure yellow product TPP-TPA was obtained by recrystallization in a DCM / n-hexane mixture (volume ratio 1:5) with a yield of 99%. 1 H NMR (400MHz, CD 2 Cl 2 ,δ): 8.11 (d, 2H, J = 0.016), 7.45 (d, 2H, J = 0.016), 7.33-7.30 (m, 4H), 7.28-7.24 (m, 4H), 7.13-7.08 (m, 7H), 7.07-7.04(m,5H), 6.98-6.93(m,4H), 6.90-6.85(m,4H), 6.80-6.75(m,4H), 4.20(s,3H), 3.79(s, 3H); 13 C NMR(100MHz, CD 2 Cl 2 ,δ): 162.95,159.34,151.19,148.96,148.05,147.03,146.84,142.76,134.81,133.82,133.40,132.92,132.75,132.60,129.64,...
Embodiment 3
[0132] Preparation and characterization of Cor-AIE points and DSPE-AIE points
[0133] Cor-PEG or DSPE-PEG (1 mg) powder and TPP-TPA (0.2 mg) were completely dissolved in THF (1 mL). After that, the THF solution was slowly added to 9 mL Milli-Q water (18.2MU) under continuous ultrasound (125W). The mixed solution was further sonicated for 1 minute, then at room temperature in N 2 While stirring, THF was removed by evaporation.
[0134] Finally, a clear solution is obtained for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com