Preparation method and application of immunomodulatory extracellular vesicles
An immunomodulatory, vesicle technology for medical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
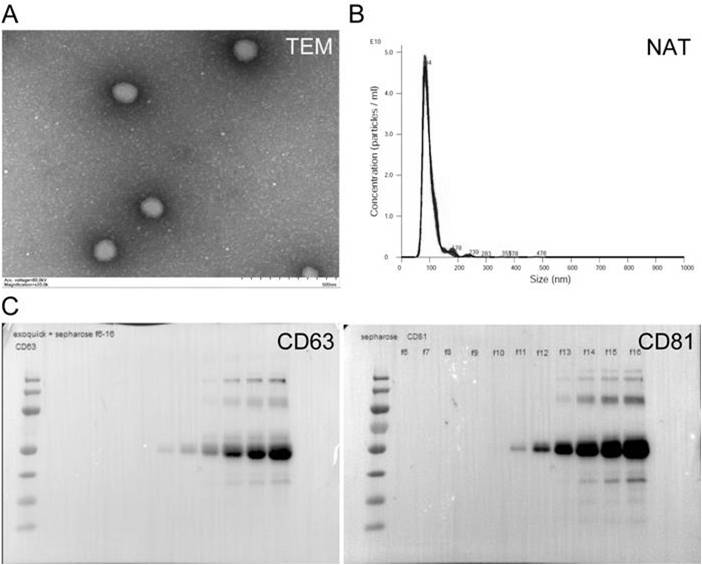
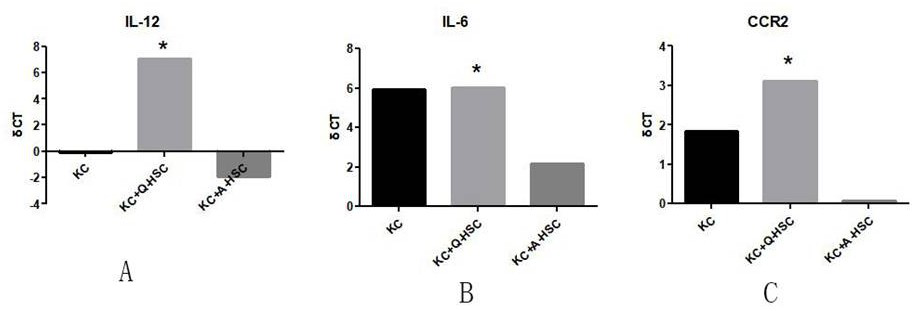
[0028] The present invention provides a preparation method of immunoregulatory small extracellular vesicles, which can activate macrophages and transform to M1 phenotype. The preparation method comprises the following steps:
[0029] (a) culturing the primary isolated and purified animal cells in a suitable culture system for a period of time, so as to accumulate an effective concentration of immunoregulatory small extracellular vesicles in the culture medium;
[0030] (b) collecting the culture medium of step (a);
[0031] (c) Isolation of immunomodulatory small extracellular vesicles from the culture medium collected in step (b).
[0032] Wherein, the suitable culture system refers to: within 3 days after the primary separation and purification, the normal interface of the two-dimensional system is cultivated; and low-sugar DMEM culture medium) to simulate the physiological microenvironment in vivo, so that the physiological behavior of cells is closer to the actual physiol...
Embodiment 1
[0071] Example 1: Isolation of Cells from Healthy Tissue
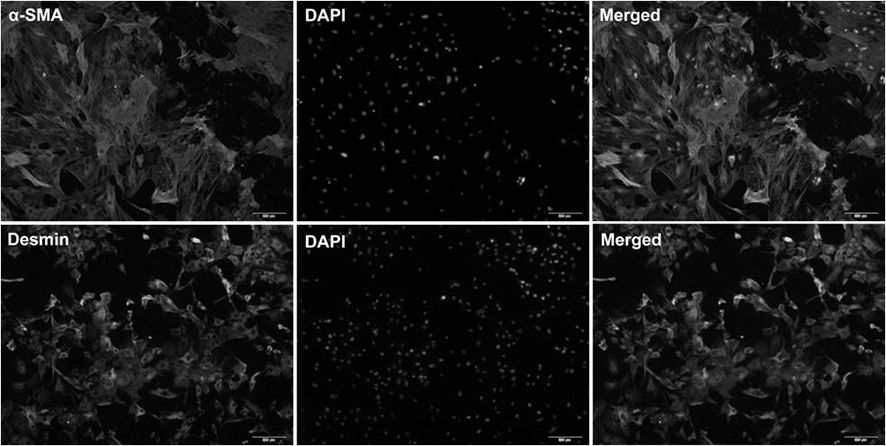
[0072] Isolation of hepatic stellate cells from healthy rat liver.
[0073] 1. Liver in situ perfusion, 0.075% type IV collagenase, digested at 37°C for 35 minutes;
[0074] 2. Separate the liver, shred it, and digest it with 0.015% type IV collagenase at 37°C for 15 minutes;
[0075] 3. Resuspend the D-Hanks, centrifuge the sample at 50g for 10min, and take the supernatant;
[0076] 4. Centrifuge at 500g for 10min to collect the precipitate;
[0077] 5. Centrifuge with a final concentration of 11-13% Nycodenz density gradient to collect cells in the middle layer;
[0078] 6. Cell counting and trypan blue staining;
[0079] 7. Plate the freshly isolated cells in a 5×10 5 Inoculate at 25cm per ml 2 In the culture flask, use Eagle's minimum basic low-glucose medium (containing 2% fetal bovine serum, 10mL / L Pen / Strep solution, 2mM Ala-Gln solution).
Embodiment 2
[0080] Example 2: Culture of isolated cells under simulated in vivo physiological conditions
[0081] For example, cells are grown by inoculating the medium at approximately 500,000 cells per milliliter.
[0082] The cells obtained in Example 1 are cultured on the ordinary two-dimensional interface until the third day, and the medium is collected; or incubated in a suitable simulated organ three-dimensional culture environment and a suitable medium. By using three-dimensional Matrigel and low-glucose culture conditions at 37°C, the physiological state in vivo is simulated. After culturing for a period of time sufficient to accumulate useful levels of immunomodulatory small extracellular vesicles, eg, up to about 6 days, the cultured cells and medium are harvested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com