Application of FGFR2b inhibiting molecule in preparation of drug for treating PAF-mediated disease
A FGF-7, molecular technology, applied in the application field of disease drugs, can solve the problems of limited improvement of patient survival rate, failure to clarify the causes of occurrence and development, inability to treat diseases, etc., to achieve easy toxic side effects and easy evaluation. , targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment 1 provides a short peptide and its pharmaceutical preparation, which is a sequence obtained by truncating 5-12 amino acids of the extracellular segment of FGFR (SEQ NO ID.52) and undergoing 0-2 point mutations. The 5-12 amino acids truncated from the extracellular segment of FGFR are spatially adjacent amino acids truncated according to the three-dimensional structure of the extracellular segment of FGFR. Specifically, the designed amino acids are represented by EWVRTD (PI9, SEQ NO ID.9), ELSGRA (PI15, SEQ NO ID.15), QDVDS (PI29, SEQ NO ID.29), VFSTTGV (PI47, SEQ NO ID. 47) as the center, the amino acid sequences are added or deleted at both ends, and the short peptides are named PI1-PI51.
[0029] Wherein, the amino acid sequence of the extracellular segment of FGFR (from the amino terminal to the carboxyl terminal) is as follows (SEQ NO ID.52):
[0030] APYWTNTEKMEKRLHAVPAANTVKFRCPAGGNPMPTMRWLKNGKEFKQEHRIGGYKVRNQHWSLIMESVVPSDKGNYTCVVENEYGSINHTYHLDVVER...
Embodiment 2
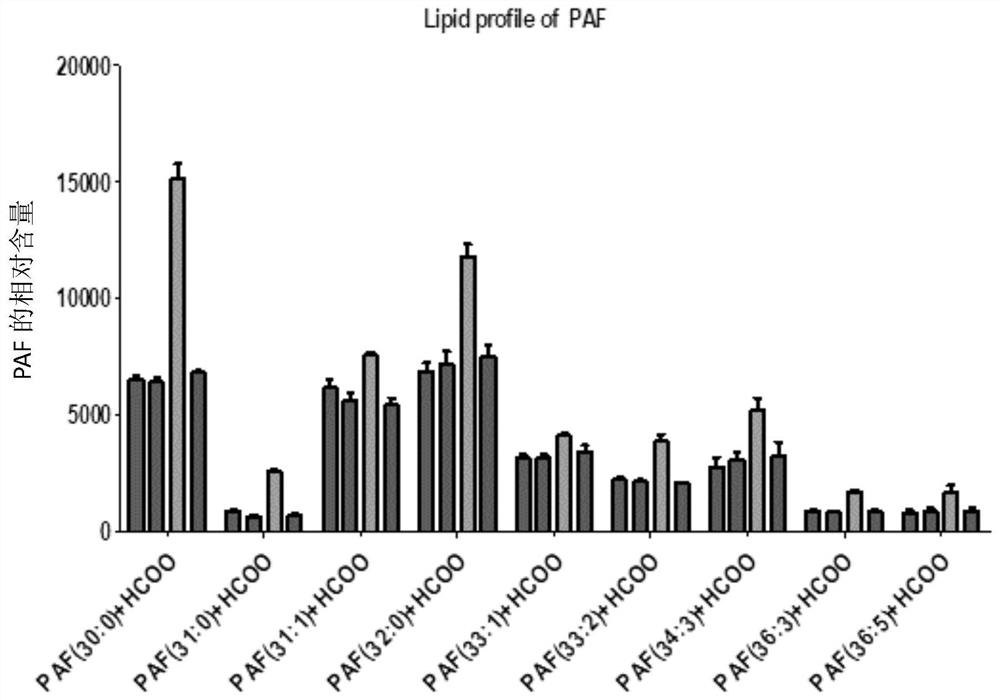
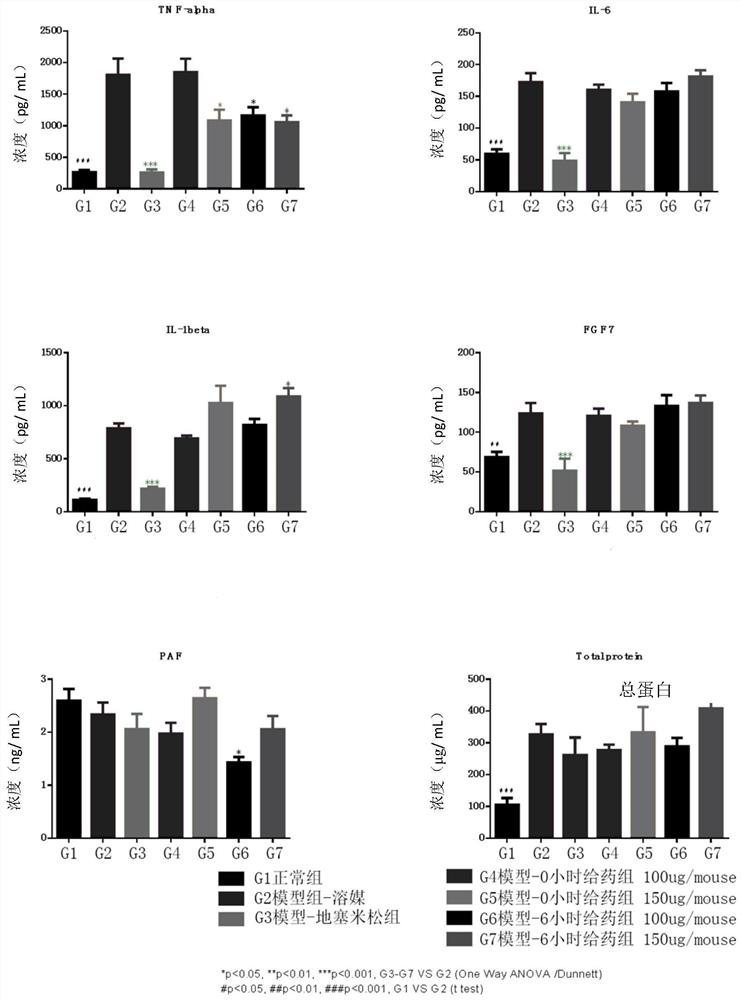
[0037] In Example 2, it was found that FGF7 regulates the expression of Platlet Activating Factor (PAF) through lipidomics research.
[0038] 1.1 Preparation of processed samples
[0039] Culture SZ95 human sebocytes, when the cells grow to 70% full, digest the cells with trypsin for 5min, discard the trypsin, add SEB-1 complete medium (SEB-1 sebocyte medium + 10% fetal bovine serum, Zenbio, USA) terminates the digestion of trypsin, blows the cells down, mixes them evenly to form a cell suspension, and counts them. The cells are grouped as shown in the table below, and each group has 3 repetitions. The primary sebocytes are plated to 10 cm for culture In a dish, add 3.7×10 to each well 5 add 10mL SEB-1 complete medium, adhere to the wall culture, change the culture medium every 48h, when the cells grow to 70% full, add serum-free medium (SEB-1 medium) to starve for 12h, discard the culture medium Base, the cells were treated as shown in Table 2, the culture medium without an...
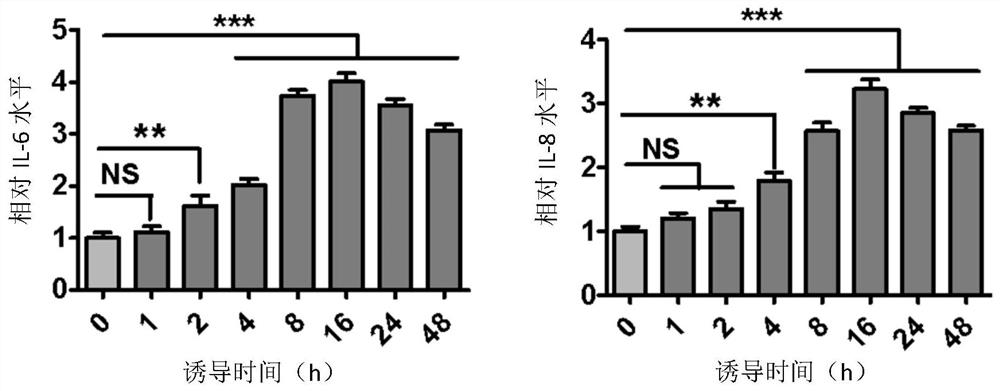
Embodiment 3
[0066] 20 μM PAF (Shanghai Yubo Biotechnology Co., Ltd.) was used to induce SZ95 human sebocytes, and then ELISA was used to detect the effect of PAF on the expression of pro-inflammatory cytokines at different times. The detection steps are as follows:
[0067] ①Sample collection: transfer the cell culture solution to EP tubes, centrifuge in a pre-cooled refrigerated centrifuge at 4°C and 12,000 rpm for 5 minutes, take the supernatant, and distribute it in EP tubes, 400 μL for each EP tube, and store in - 20°C refrigerator, take it out and let it melt at room temperature before use;
[0068] ②According to the instructions of IL-6ELISA detection kit and IL-8ELISA detection kit of Beijing Sizhengbai Biotechnology Co., Ltd., take the kit and experimental samples stored at 4°C to room temperature for 30 minutes, and equilibrate to room temperature;
[0069] ③Add the sample to be tested into the corresponding enzyme-labeled well, 100 μL per well, seal the reaction well with seali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com