Low-interfering RNA (ribonucleic acid) medicine for treating chronic hepatitis B and hepatic fibrosis
A technology of liver fibrosis and small interference, applied in DNA/RNA fragments, gene therapy, drug combination, etc., can solve problems such as easy recurrence and drug resistance mutation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 Recombinant vector construction and recombinant virus packaging
[0040] Anneal the oligonucleotide (i.e. the DNA complementary primer corresponding to the small interfering RNA of the present invention, see sequence 3 and sequence 4 in the sequence listing, synthesized by the applicant according to the sequence of the small interfering RNA) to form a double-stranded fragment and clone it into a linearized vector In the pSC-H1-empty vector, the recombinant plasmid pSC-H1-shRNA ( figure 1 ). The brief operation process is as follows:
[0041] Annealed oligonucleotides
[0042] Dissolve oligonucleotides in DNase-free and RNase-free sterile water to a final concentration of 3 mg / mL. The specific calculation formula is as follows:
[0043] The amount of water needed to obtain the final concentration of the oligonucleotide solution=(oligonucleotide quality μg×10 -3 ) / final concentration (X mg / mL)
[0044] To obtain an oligonucleotide solution with a final...
Embodiment 2
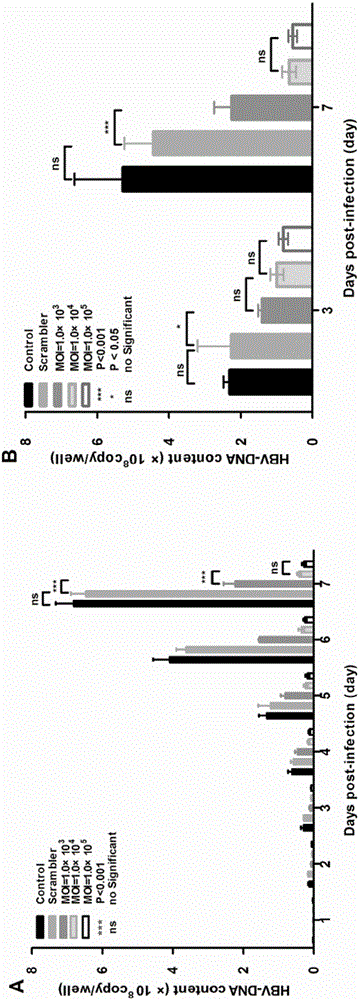
[0074] Embodiment 2 in vitro antiviral experiment
[0075] 2.1 Recombinant adeno-associated virus AAV2-shRNA infection of HepG2.2.15
[0076] (1) with 4×10 5 Density of cells / well HepG2.2.15 cells were seeded in a six-well plate, 2 mL of culture medium was added to each well, cultured to a confluence of about 70%, and then infected. Medium components: high glucose DMEM+10%FBS+1% double antibody+G418 (200μg / mL);
[0077] (2) Before infection, aspirate the medium in the wells of the six-well plate, and wash the cells 2-3 times with PBS (1 mL PBS / well). Add MOI=10 respectively 3 , MOI=10 4 , MOI=10 5 The virus (the virus was prepared with PBS to an equal volume of 500 μL) was incubated at 37° C. in 5% CO2 for 3 hours. Aspirate the virus liquid, add 2 mL of complete medium to each well to continue the culture, harvest the supernatant after 24 hours, and add 2 mL of fresh medium to the six-well plate to continue the culture. Subsequently, the supernatant was aspirated every ...
Embodiment 3
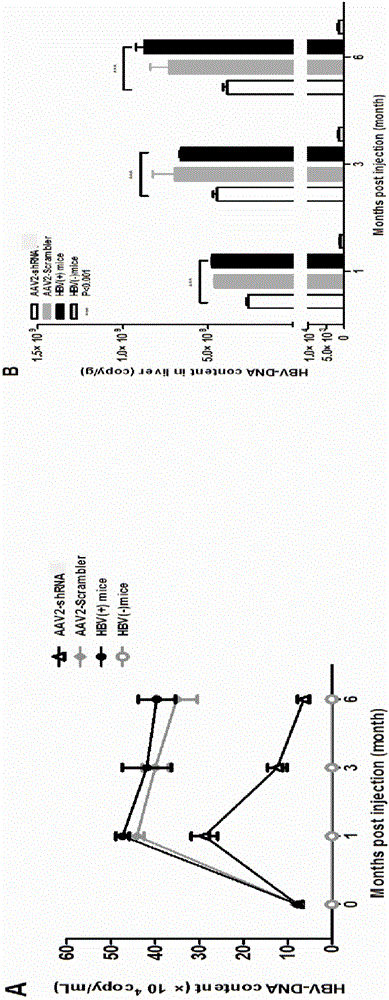
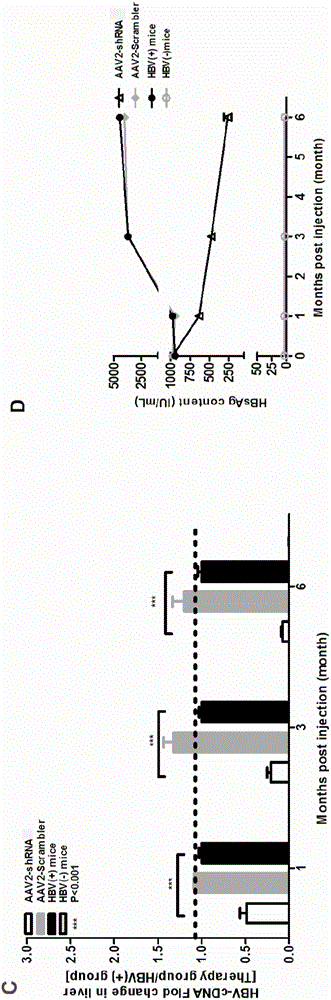
[0088] Embodiment 3 in vivo antiviral experiment
[0089] Press 1×10 11 The dose of copy / mouse will inject AAV2-shRNA tail vein into the mouse model of hyperhepatophilic AAV8-1.2HBV hepatitis B chronic infection for 1 month to construct the treatment model, and take serum samples and tissue samples at each time point, Measure each index to evaluate the treatment model.
[0090] The specific scheme is as follows: Dilute AAV8-1.2HBV with PBS to 2×10 11 copy / 200μL PBS, that is, each C57BL / 6 mouse was injected with a volume of 200μL virus, and the virus dose was 2×10 11 copy / only; put the successfully injected mice back into the mouse cage to continue feeding, the feeding conditions were 25°C, humidity 30%-60%, given 12 hours of light and 12 hours of darkness every day. At one month of modeling, the blood of the mice was taken to detect HBV DNA, HBsAg and HBeAg levels, and the mice that were positive for all three were used as mouse models of chronic hepatitis B infection with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com