Application of N-substituted phenyl-2-pyridone compounds or pharmaceutically acceptable salts thereof in treatment of pulmonary fibrosis
A pulmonary fibrosis and compound technology, applied in the field of medicine, can solve the problem of irreversible pulmonary fibrosis, and achieve the effect of improving the degree of pulmonary fibrosis, improving safety and protecting the lungs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthetic method of compound P adopts prior art, and synthetic general formula and process are as follows:
[0037]
[0038] Compound 1 (20 g), iodobenzene derivative 2 (1.5 equiv.), potassium carbonate (1.1 equiv.), cuprous chloride (10 mol%) and DMF (300 mL) were placed in a 500 mL reaction flask under argon protection , 150 ℃ reflux reaction for 10 hours. After the reaction was completed, it was cooled to room temperature, filtered with suction, the filter cake was washed three times with DMF, the filtrates were combined, and activated carbon was added to decolorize. The decolorized solution was evaporated to dryness under reduced pressure, 100 mL of 10% acetic acid was added, and the mixture was stirred at 70° C. for 30 minutes. After standing for layers, the upper aqueous phase was taken, the lower oily substance was extracted twice with 10% acetic acid, and the aqueous phases were combined. The aqueous phase was adjusted to pH 13 by adding sodium hydroxid...
Embodiment 2
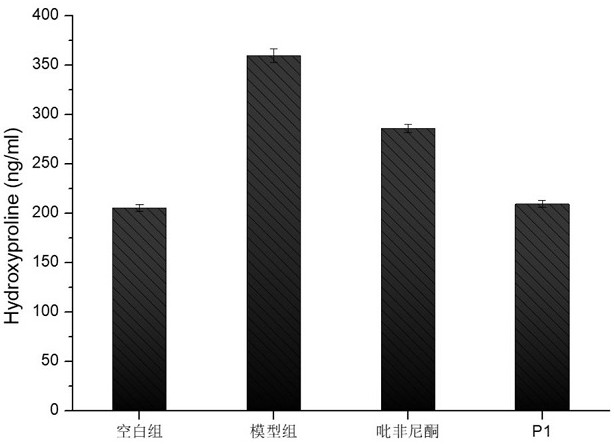
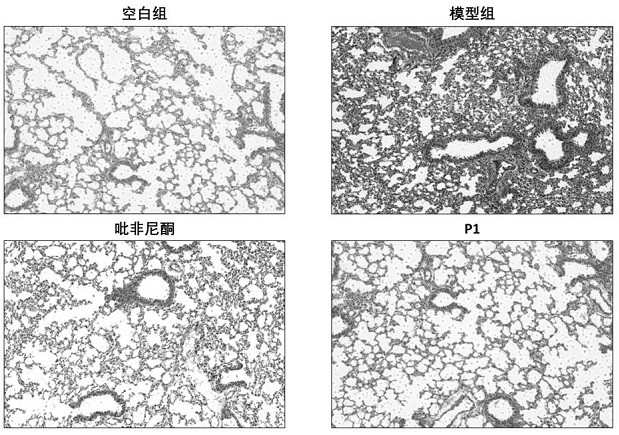
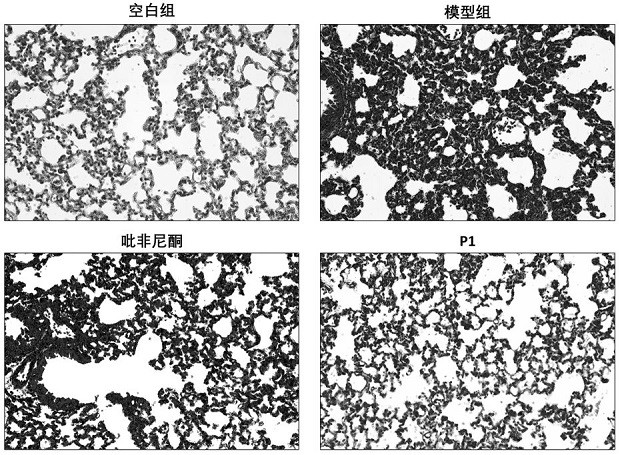
[0071] Animal grouping, surgical modeling and administration: Mice were randomly divided into blank control group, model control group, pirfenidone treatment group, and new drug treatment group. Mice were acclimated for 3 days (weight about 20 g), and the model was operated on the 4th day. The method was as follows: after weighing the mice, anesthetized by intraperitoneal injection of sodium pentobarbital, put the anesthetized mice in a supine position, and fix the limbs and head of the mice with a mouse plate, with the head high and the feet low, fully exposing the neck skin. The anterior cervical area of the mice was routinely depilated and disinfected, and a median longitudinal incision was made, and the muscles and tissues were bluntly separated until the trachea was exposed. Bleomycin solution (2mg / ml) was prepared with sterile normal saline, the syringe was inserted into the trachea through the tracheal cartilage ring gap towards the heart end, and after the air was wi...
Embodiment 3
[0087] The test data of other compounds are given in the form of the following table, wherein, the test process and method refer to Example 2.
[0088] Table 1 Properties data table of other compounds
[0089]
[0090]
[0091]
[0092] It can be seen from the experimental data in the table that the degree of fibrosis of the lung tissue has been relieved to a great extent after the treatment with the above N-substituted phenyl-2-pyridone compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com