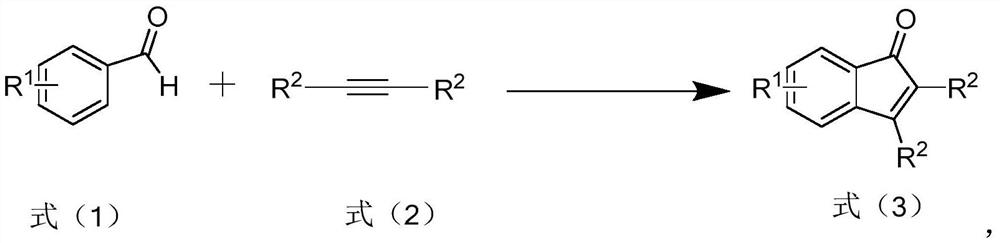
Preparation method of polysubstituted indanone derivative
A compound and reaction technology, applied in the field of multi-substituted indanone derivatives and its preparation, can solve problems such as complex operation, difficult preparation of raw materials, and many reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0028] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0029] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
Embodiment 1
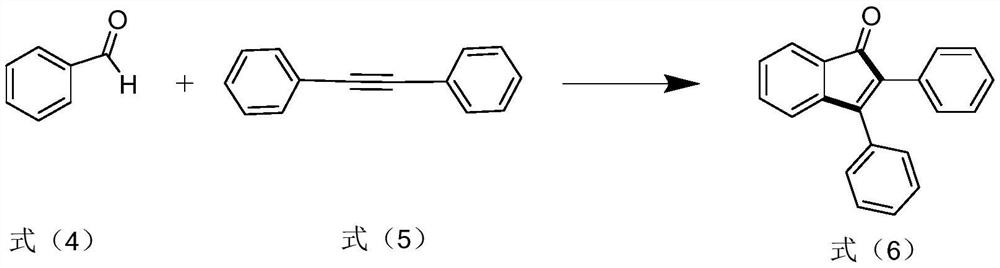
[0031]
[0032] Add 2.0mmol of the compound of formula (4), 0.5mmol of the compound of formula (5), and 2.0mL of acetonitrile into a 10mL reaction tube, place it in an oil bath at 140°C under the irradiation of a 250W UV lamp with a maximum wavelength of 365nm, and react for 48h . Stop the reaction, cool to room temperature, add dichloromethane to dilute, extract with water three times, dry the organic phase with anhydrous sodium sulfate, filter, and the filtrate is separated by column chromatography to obtain 120 mg of the compound of formula (6), with a yield of 85% and a purity of 99.5 %. The NMR characterization of this compound is as follows: 1 HNMR (400MHz, CDCl 3 )δ7.59(d,J=7.0Hz,1H),7.46–7.33(m,6H),7.31–7.22(m,6H),7.15(d,J=7.2Hz,1H); 13 C NMR (100MHz, CDCl 3 )δ196.5, 155.3, 145.2, 134.9, 133.4, 132.7, 132.4, 130.7, 130.0, 129.3, 128.9, 128.8, 128.5, 128.0, 127.7, 123.0, 121.2.
Embodiment 2
[0034] Add 2.0mmol of the compound of formula (4), 0.5mmol of the compound of formula (5), and 2.0mL of acetonitrile into a 10mL reaction tube, place it in an oil bath at 140°C under the irradiation of a 25W UV lamp with a maximum wavelength of 365nm, and react for 48h . Stop the reaction, cool to room temperature, add dichloromethane to dilute, extract with water three times, the organic phase is dried with anhydrous sodium sulfate, filtered, and the filtrate is separated by column chromatography to obtain 11 mg of the compound of formula (6), with a yield of 8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com