A kind of triarylamine compound and its organic electroluminescent device
A technology of compound and triarylamine, which is applied in the field of triarylamine compound and its organic electroluminescent device, can solve the problems of material type, thickness, and difficulty in matching the refractive index, so as to improve light extraction efficiency, high glass transition temperature, and improve luminescence The effect of efficiency and service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] The synthesis of embodiment 1 compound 2
[0120]
[0121] Toluene solvent was added into the reaction flask, followed by 2-(4-bromophenyl)benzoxazole (54.82g, 200mmol), 4-aminobiphenyl (37.23g, 220mmol), sodium tert-butoxide (57.66g, 600mmol), after vacuuming and replacing with nitrogen for three times, add Pd(OAc) 2 (0.9g, 4.0mmol), after vacuumizing and nitrogen replacement three times, add P(t-Bu) 3 (6.4mL of 1.0M toluene solution, 6.4mmol), and nitrogen replacement was performed three times, and the mixture was refluxed for 2h under nitrogen atmosphere. After the reaction was stopped, the mixture was cooled to room temperature, filtered through diatomaceous earth to obtain the filtrate, and the filtrate was concentrated , adding 20mL of methanol, standing for recrystallization, and filtering to obtain intermediate 2-1 (62.34g, 86%), and the solid purity was detected by HPLC ≧98.9%.
[0122] Add toluene solvent to the reaction flask, and then add intermediate 2...
Embodiment 2
[0124] The synthesis of embodiment 2 compound 22
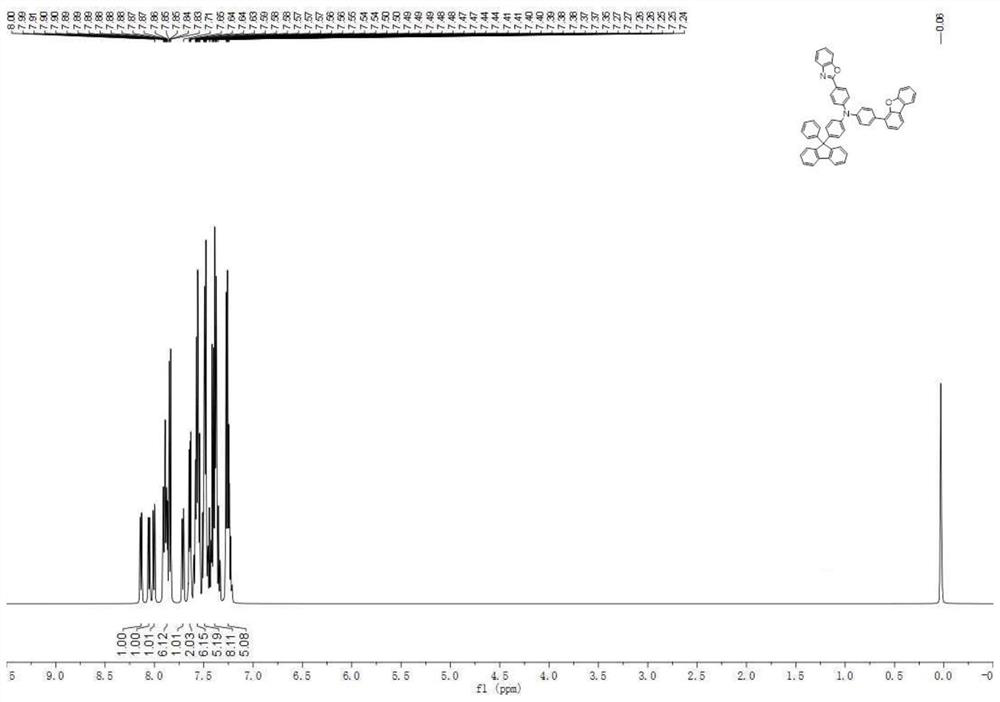
[0125] In Example 1, the raw material 4-aminobiphenyl is replaced with 4-(benzo[D]oxazol-2-yl)aniline of equimolar amount, and 2-(4-bromophenyl)benzoxazole is replaced with etc. The molar amount of 4-(4-bromophenyl)-dibenzofuran was synthesized according to the synthesis method of compound 2 to obtain compound 22 (59.21 g, 77%), and the solid purity was detected by HPLC ≧99.9%.
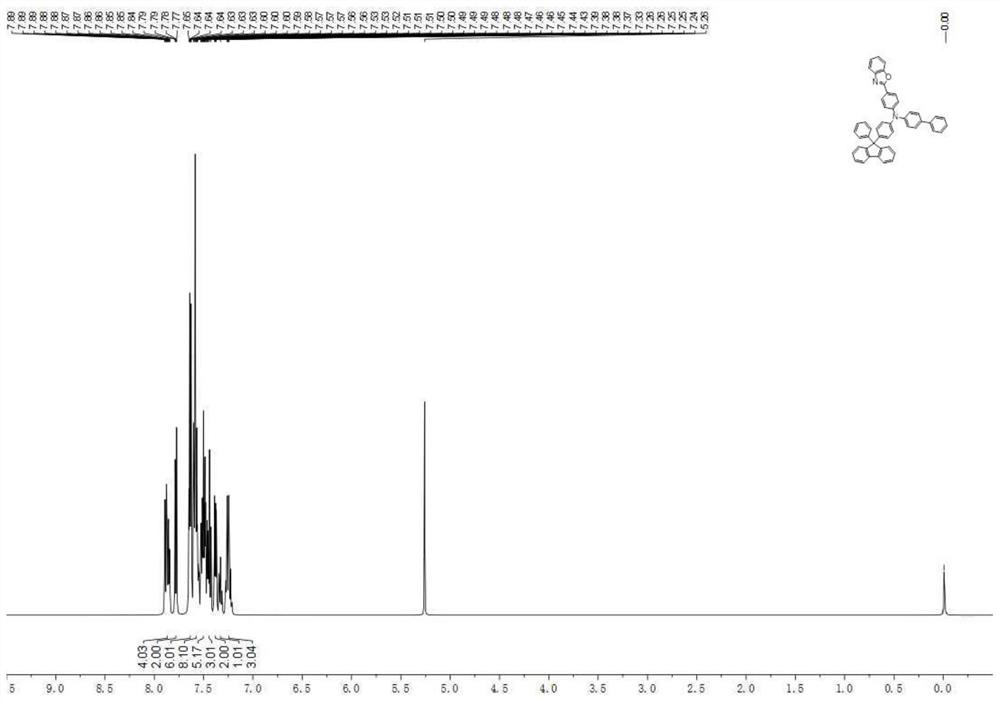
[0126] Mass Spectrum m / z: 768.2784 (theoretical value: 768.2777). Theoretical element content (%)C 56 h 36 N 2 o 2 : C, 87.48; H, 4.72; N, 3.64; O, 4.16. Measured element content (%): C, 87.51; H, 4.71; N, 3.63; O, 4.15. 1 H-NMR (500MHz, CDCl3) (δ, ppm): 8.14 (dd, J = 7.5, 1.5Hz, 1H), 8.05 (dd, J = 7.4, 1.5Hz, 1H), 8.00 (d, J = 1.5Hz ,1H),7.92–7.82(m,6H),7.71(s,1H),7.64(dd,J=5.6,3.5Hz,2H),7.60–7.53(m,6H),7.52–7.46(m,5H ), 7.45–7.33(m,8H), 7.29–7.21(m,5H). The above results confirmed that the obtained product was the target product.
Embodiment 3
[0127] The synthesis of embodiment 3 compound 28
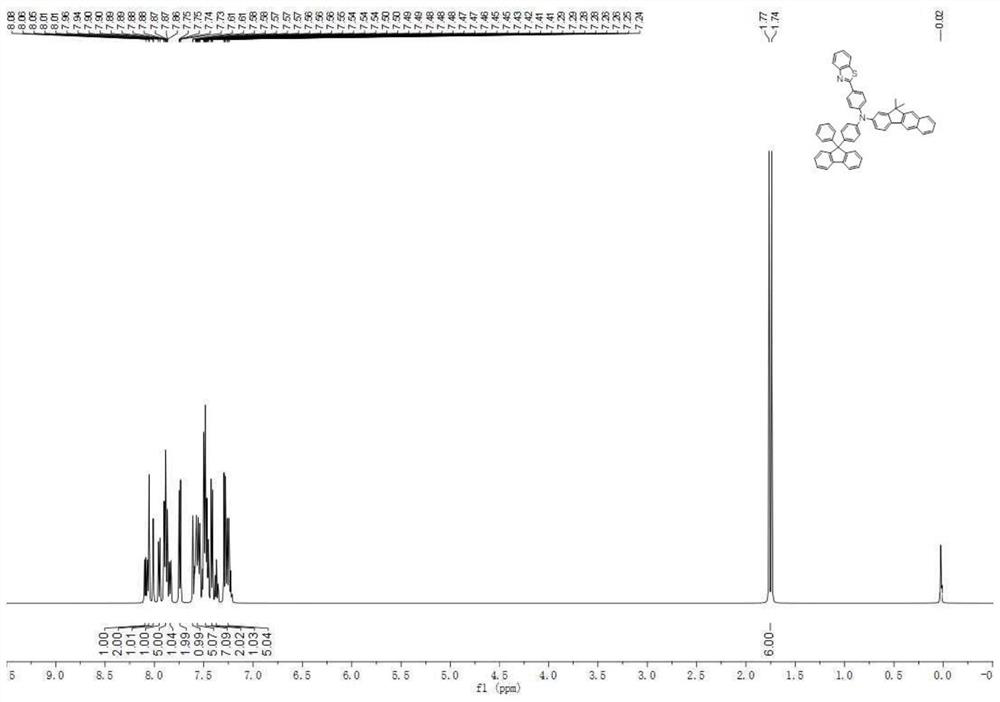
[0128] The raw material 4-aminobiphenyl in Example 1 was replaced by an equimolar amount of 4-(benzo[D]oxazol-2-yl)aniline, and compound 28 (57.59g, 80%) was obtained according to the synthesis method of compound 2 , HPLC detection solid purity ≧ 99.9%.
[0129] Mass Spectrum m / z: 791.2586 (Theoretical: 719.2573). Theoretical element content (%)C 51 h 33 N 3 o 2 : C, 85.10; H, 4.62; N, 5.84; O, 4.45. Measured element content (%): C, 85.07; H, 4.63; N, 5.85; O, 4.45. The above results confirmed that the obtained product was the target product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com