Separation and purification method of adeno-associated virus
A technology for separation and purification of viruses, applied in the field of separation and purification of adeno-associated viruses, can solve problems such as tissue tropism affecting transduction efficiency, and achieve the effects of convenient purification of AAV, high purification efficiency and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation and detection of antibodies
[0042] 1. Antibody preparation
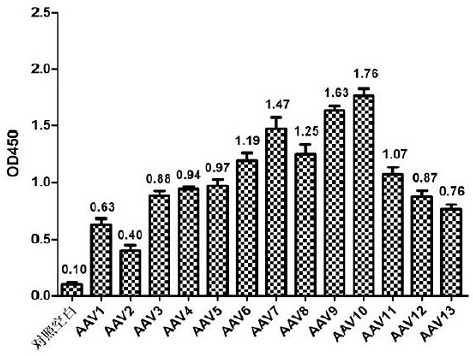
[0043] The antigen used in the present invention is based on the VP1 of AAV8, compares AAV1-13, removes most of the non-homologous sequences, obtains the VPx sequence, constructs it on the pET-21 vector, expresses and purifies the representative, and obtains the small Mouse monoclonal antibody.
[0044] 1. Preparation of Monoclonal Antibody
[0045] 1.1 Immunization of mice:
[0046] Immunization of BALB / c mice The immunization of BALB / c mice was carried out according to the conventional method. One week after the second immunization, 200 μL blood was collected by tail-docking vacuum method for antibody detection. After the second immunization, the mice were rested for two weeks for booster immunization. , fused after three days.
[0047] 1.2 Cell Fusion:
[0048] 50% PEG with a molecular weight of 1450 was used as a fusion agent for cell fusion, and the cell fusion was carried out...
Embodiment 2
[0069] Example 2 Separation and purification method of adeno-associated virus
[0070] 1. Antibody Conjugation Activated Media Step
[0071] Referring to the NHS-activated Sepharose 4 Fast Flow manual, the AAV antibody prepared in Example 1 was coupled to Sepharose 4 Fast Flow to prepare an affinity filler.
[0072] 2. Affinity purification step for AAV
[0073] 1) Equilibration: equilibrate the packed column with 8CV of 10 mM Tris-HCl, 150 mM NaCl, pH7.5 solution.
[0074] 2) Loading: Freeze-thaw, lyse and centrifuge HEK293 cells packaged with AAV1~13 respectively, filter through 0.22 μm, and then load the sample to a well-balanced chromatographic column. The loading capacity of the sample does not exceed 1E+14 vg / mL filler .
[0075] 3) Washing 1: After loading the sample, equilibrate with 8CV of 10 mM Tris-HCl, 150 mM NaCl, pH7.5 solution.
[0076] 4) Washing 2: Use 8CV of 10 mM Tris-HCl, 1 M NaCl, pH7.5 solution to wash out impurities.
[0077] 5) Wash 2: Equilibrate ...
Embodiment 3
[0082] Example 3 Separation and purification method of adeno-associated virus
[0083] 1. Antibody Conjugation Activated Media Step
[0084] Referring to the NHS-activated Sepharose 4 Fast Flow manual, the AAV antibody prepared in Example 1 was coupled to Sepharose 4 Fast Flow to prepare an affinity filler.
[0085] 2. Affinity purification step for AAV
[0086] 1) Equilibration: equilibrate the packed column with 5CV of 8 mM Tris-HCl, 130mM NaCl, pH=7.3 solution.
[0087] 2) Loading: Freeze-thaw, lyse and centrifuge HEK293 cells packaged with AAV1~13 respectively, filter through 0.22 μm, and then load the sample to a well-balanced chromatographic column. The loading capacity of the sample does not exceed 1E+14 vg / mL filler .
[0088] 3) Washing 1: After loading the sample, equilibrate with 5CV of 8 mM Tris-HCl, 130mM NaCl, pH=7.3 solution.
[0089] 4) Washing 2: Use 8 mM Tris-HCl, 0.8M NaCl, pH=7.3 solution to wash away impurities.
[0090] 5) Washing 2: Equilibrate the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com