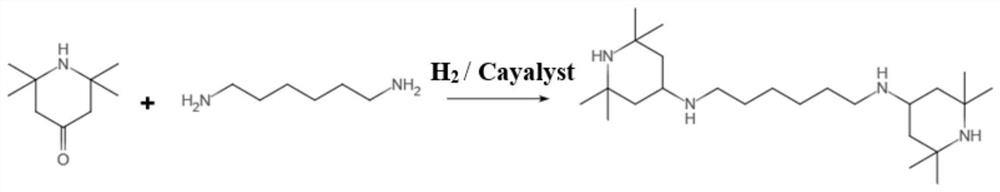
Synthesis process of hexamethylenediamine piperidine
A technology of hexamethylenediaminepiperidine and synthesis process, which is applied in the synthesis process of hexamethylenediaminepiperidine, the intermediate field of hindered amine light stabilizers, can solve the increase of production cost and difficulty, does not have the by-product piperidinol, It is not suitable for industrial production and other problems, to meet the requirements of simplifying equipment and processes, improve recycling efficiency, and achieve the effect of recycling and applying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The whole chemical reaction is as attached figure 1 The chemical reaction equation shown is shown.
[0044] Add 500g of 2,2,6,6-tetramethyl-4-piperidone and 150g of 1,6-hexamethylenediamine into the reactor;
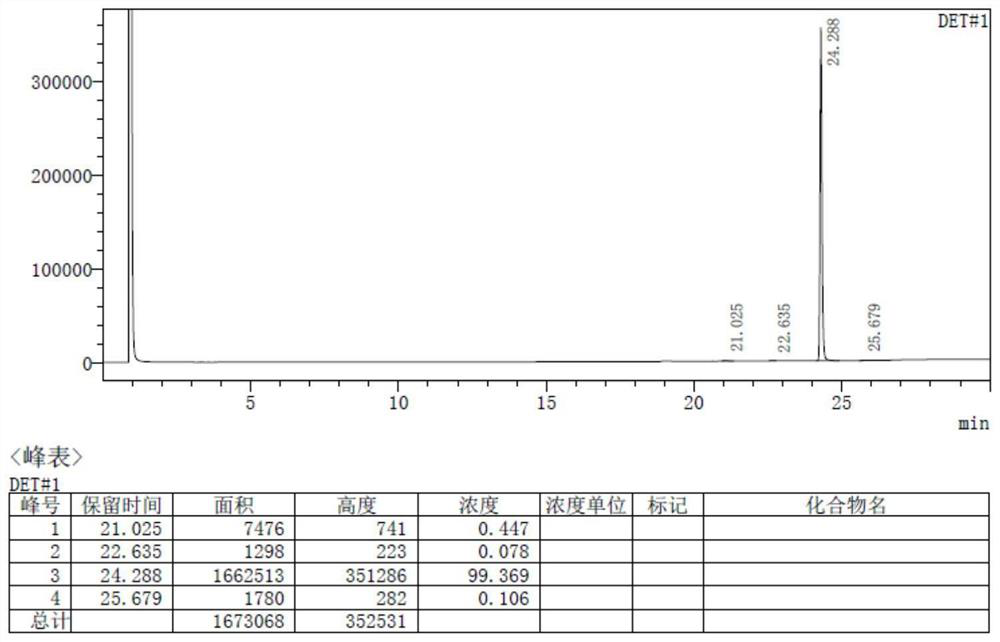
[0045] Use the vacuum circulation pump to provide negative pressure, slowly increase the vacuum degree to -0.085MPa, and start to slowly increase the temperature. When the temperature rises to 95°C, observe the moisture in the system and start the timing reaction; The vacuum degree reaches -0.085MPa. At this time, use nitrogen to break the air, then add anhydrous magnesium sulfate with 5% of the total weight of reactants to the system, and re-connect the negative pressure to continue the heat preservation reaction. Content of Schiff's base, when the content of Schiff's base is ≥98%, the reaction time is 3 hours, after the reaction is completed, the nitrogen is vented, and the Schiff's base is stored under liquid nitrogen;
[0046] Put the Schiff's base liquid in...
Embodiment 2
[0049] Add 440g of 2,2,6,6-tetramethyl-4-piperidone and 150g of 1,6-hexamethylenediamine into the reactor;
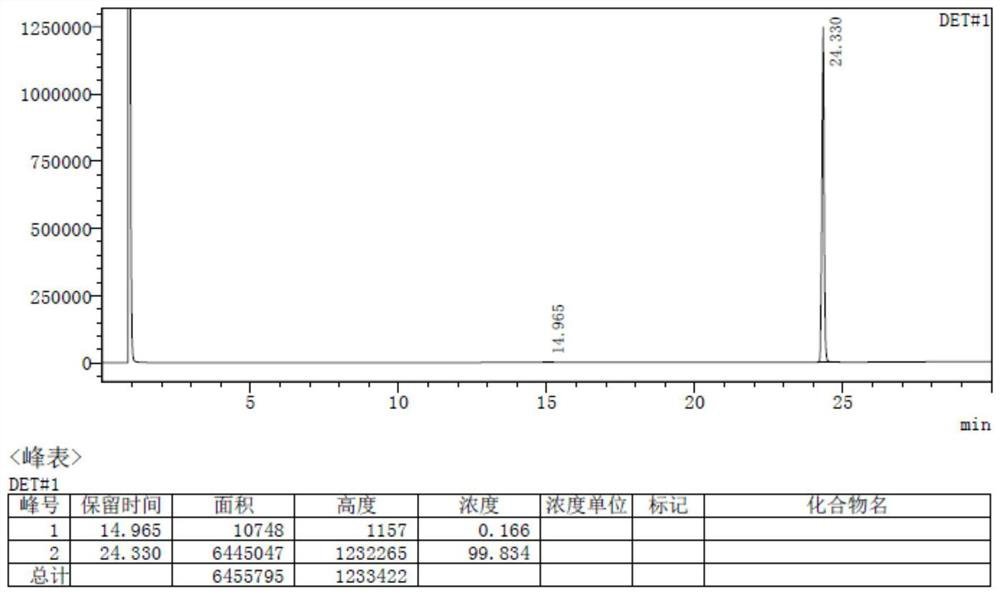
[0050] Use a vacuum circulating water pump to provide negative pressure, slowly increase the vacuum degree to -0.085MPa, and start to slowly increase the temperature. When the temperature rises to 60°C, observe the moisture in the system and start the timing reaction; When the vacuum reaches -0.085MPa, use nitrogen to break the air, then add anhydrous sodium sulfate with 10% total weight of reactants to the system, and connect to negative pressure. During this period, use GC to monitor the content of Schiff base. When the content of Schiff base is ≥ When 98%, the reaction is finished, the reaction time is 6 hours, the nitrogen is broken, and the Schiff base is stored under liquid nitrogen.
[0051] Put the Schiff's base liquid into the autoclave, and according to the quality of the Schiff's base liquid, put in 5% of its weight Raney nickel catalyst, cover the reactor, r...
Embodiment 3
[0054] The details are the same as in Example 2, except that the degree of vacuum reaches -0.1 MPa when the vacuum pumping operation is carried out.
[0055] The total yield of hexamethylenediamine piperidine based on 1,6-hexamethylenediamine is more than or equal to 98.7%, and the purity is 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com