Synthesis method of Tucatinib and intermediate product thereof
A synthesis method and compound technology, applied in the field of synthesis of tucatinib and its intermediate products, can solve problems such as expensive, difficult, and scaled-up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
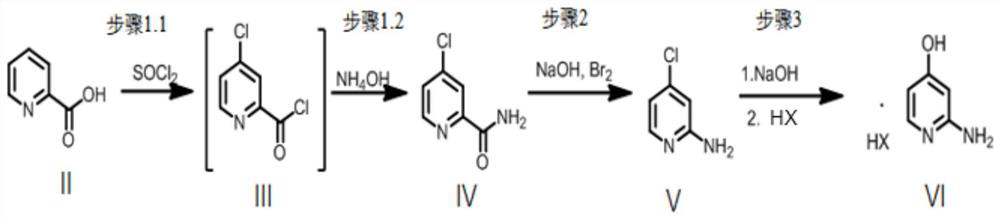
[0078] Embodiment 1: the synthetic route of the hydrochloride of compound as shown in VI
[0079] First, in a 500 mL flask, the compound represented by formula II (10.00 g, 81.23 mmol) and sodium bromide (420 mg, 0.05 eq) were added, and thionyl chloride (45 mL) was added dropwise. The resulting mixture was slowly heated to reflux, during which time a vigorous evolution of sulfur dioxide occurred. After 16 h at reflux, the solution was cooled to room temperature, diluted with toluene (50 mL), and concentrated in vacuo. This process is repeated twice. Then ammonia water (20 vol, 180 mL) was slowly added dropwise to the obtained yellow crude product at 0°C. Stir at room temperature for 18 h, ice-bath, precipitate a white solid, then filter the suspension to obtain a crude light white filter cake, wash with water to obtain a pure product. The filter cake was vacuum dried to obtain 10.8 g of the compound represented by formula IV as a white powder. The yield is 85%, the purity...
Embodiment 2
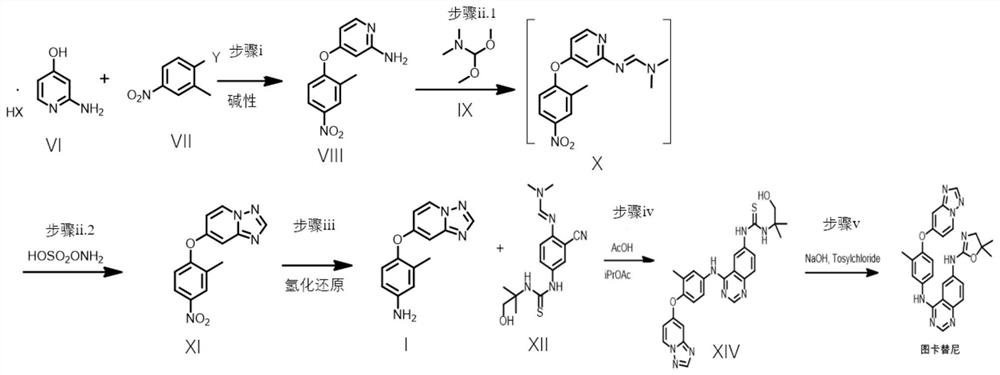
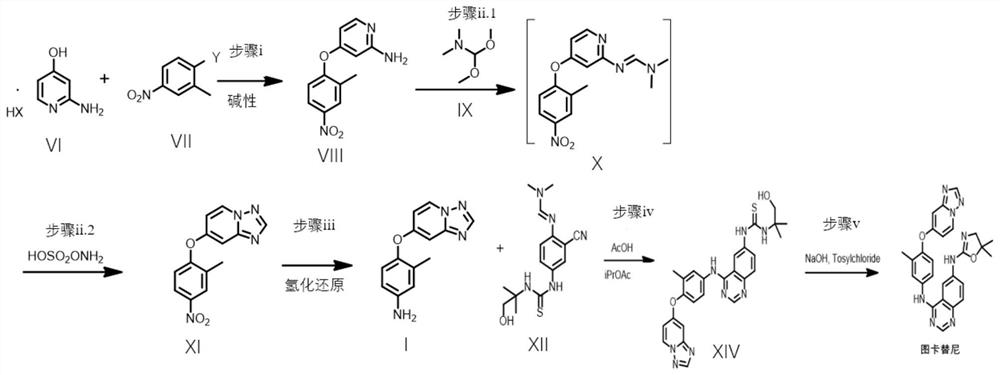
[0085] Embodiment 2: the synthetic route of Tucatinib
[0086] In a 250mL flask, add the hydrochloride (5g, 34.4mmol, 1.0 equivalent) of the compound shown in formula VI, and 1-fluoro-2-methyl-4-nitrobenzene (5.32g, 34.4mmol, 1.0 equivalent) , DMF (100 mL), triethylamine (5.2 g, 51.6 mmol, 1.5 eq) and potassium hydroxide (2.9 g, 51.6 mmol, 1.5 eq). After the mixture was stirred at room temperature for 12-16 h, triethylamine (1.5 eq) and potassium hydroxide (1.5 eq) were added to react according to the remaining amount of the starting material until HPLC analysis showed that only less than 1% of the starting material remained. The total reaction time is less than 18h. The reaction mixture was poured into 500 g of ice and stirred for 2 h, a yellow precipitate formed. Filtration yielded a crude light yellow filter cake. The pure product was then obtained after recrystallization of the filter cake. The filter cake was dried under vacuum to obtain 5.64 g of the compound represe...
Embodiment 3
[0092] Example 3: The difference between this example and Example 2 lies in the synthetic route of the compound shown in formula VIII.
[0093] In a 25mL flask, add the hydrochloride (0.29g, 2mmol, 1.0 equivalent) of the compound shown in formula VI, and 1-fluoro-2-methyl-4-nitrobenzene (0.47g, 3mmol, 1.5 equivalent) , DMSO (6 mL), triethylamine (0.3 g, 3 mmol, 1.5 eq) and potassium hydroxide (0.17 g, 3 mmol, 1.5 eq). After the mixture was stirred at 70° C. for 8-12 h, triethylamine (1.5 eq) and potassium hydroxide (1.5 eq) were added according to the remaining amount of the starting material until HPLC analysis showed that only less than 1% of the starting material remained. The total reaction time does not exceed 18h. The reaction mixture was poured into 50 g of ice and stirred for 2 h, a yellow precipitate formed. Filtration yielded a crude light yellow filter cake. Then the filter cake was recrystallized and filtered to obtain 0.32 g of the compound represented by formu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com